FROM ELEMENTS TO COMPOUNDS
0.0(0)
Card Sorting
1/100
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
1
New cards
elements
- substances that are pure in their nature
- no contaminants, nothing else is mixed in it
- are composed of a single atom
- not just limited to single elements (i.e. Oxygen [O2] is still an element even if 2 oxygen is involved in the molecule)
- no contaminants, nothing else is mixed in it
- are composed of a single atom
- not just limited to single elements (i.e. Oxygen [O2] is still an element even if 2 oxygen is involved in the molecule)
2
New cards
compounds
- pure substances that are composed of elements in definite proportions
- heterogenous mixture of atoms
- contains 2 or more kinds of atoms
- ex: water (H2O -> 2 hydrogen, 1 oxygen = 2 kinds of atoms)
- heterogenous mixture of atoms
- contains 2 or more kinds of atoms
- ex: water (H2O -> 2 hydrogen, 1 oxygen = 2 kinds of atoms)
3
New cards
Dalton's Atomic Theory
- 1808 school teacher and chemist
- "Each element is made up of particles called atoms"
- atoms of a given element are identical
- chemical reactions involve reorganizations of the atoms - changes in the way they are bound together
- but atoms are not chemically changed
- "Each element is made up of particles called atoms"
- atoms of a given element are identical
- chemical reactions involve reorganizations of the atoms - changes in the way they are bound together
- but atoms are not chemically changed
4
New cards
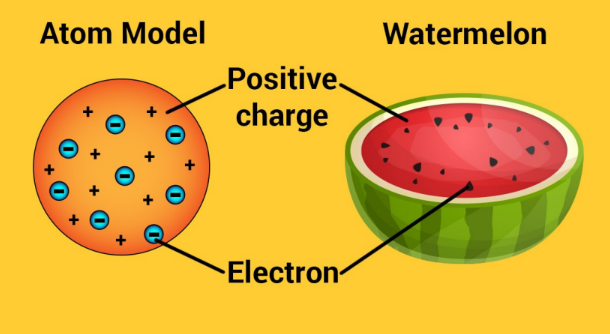
J.J Thomson's Atomic Model
- 1897, British Physicist
- discovered the electron in his cathode ray tube
- plum pudding model of the atom
- there's a big mass of positively charged area/environment where electrons are embedded
- discovered the electron in his cathode ray tube
- plum pudding model of the atom
- there's a big mass of positively charged area/environment where electrons are embedded
5
New cards
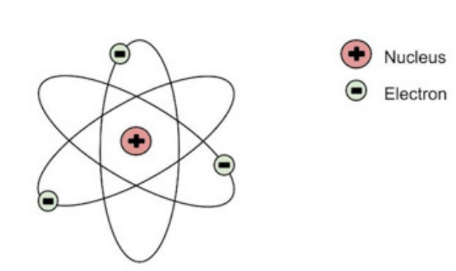
Ernest Rutherford's Atomic Model
- 1908
- performed the gold foil experiment
- atom is made up of mostly empty space and contains a positively charged nucleus in the center
- atom is unstable bc of the movement of the electron orbiting around the positively charged nucleus
- no neutrons yet
- protons and electrons are only subatomic particles here
- performed the gold foil experiment
- atom is made up of mostly empty space and contains a positively charged nucleus in the center
- atom is unstable bc of the movement of the electron orbiting around the positively charged nucleus
- no neutrons yet
- protons and electrons are only subatomic particles here
6
New cards
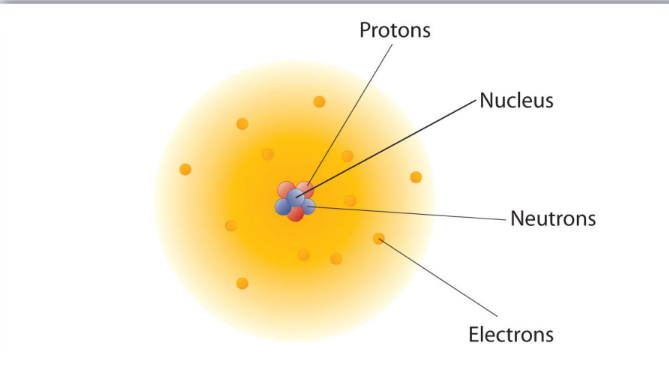
20th century
- development of mass spectrophotometers lead to the discovery of neutrons
- neutrons are in nucleus together w/ the protons
- present atomic model where nucleus is composed of protons and neutrons
- electrons surround nucleus
- neutrons are in nucleus together w/ the protons
- present atomic model where nucleus is composed of protons and neutrons
- electrons surround nucleus
7
New cards
periodic table of elements
- arrangement of elements by property
- there are trends of several properties in each element
- there are trends of several properties in each element
8
New cards
periods
- atomic size/radius decreases as you move across _____ (from left to right)
- ionization energy increases
- electronegativity increases
- ionization energy increases
- electronegativity increases
9
New cards
groups
- atomic size/radius increases as you move down ______
- ionization energy decreases
- electronegativity decreases
- ionization energy decreases
- electronegativity decreases
10
New cards
periods' atomic size/radius
- decreases as you move across
- number of electrons and protons increase
- increased number of protons increases nuclear charge, pulling the electrons closer to the nucleus
- increased nuclear charge increases its pool
- atom decreases bc of increase in protons and nuclear charge
- composed of positively charged protons and neutral neutrons
- electrons get attracted to nucleus, compressing the atom
- number of electrons and protons increase
- increased number of protons increases nuclear charge, pulling the electrons closer to the nucleus
- increased nuclear charge increases its pool
- atom decreases bc of increase in protons and nuclear charge
- composed of positively charged protons and neutral neutrons
- electrons get attracted to nucleus, compressing the atom
11
New cards
groups' atomic size
- (number of electrons) increase moving down
- more electrons in more principal energy levels
- atom increases
- nucleus can't attract electrons on the outer shell due to inner electrons
- shielding occurs and outer electrons can't feel attracting pull of protons
- repulsions to electrons from both being negatively charged
- more electrons in more principal energy levels
- atom increases
- nucleus can't attract electrons on the outer shell due to inner electrons
- shielding occurs and outer electrons can't feel attracting pull of protons
- repulsions to electrons from both being negatively charged
12
New cards
ionization energy
- energy needed to overcome reaction of the nuclear charge and remove an electron from gaseous atom
- increases across a period and decreases down a group
- related to atomic size
- outer electrons feel lesser pull from the nucleus bc of the inner electrons
- repulsion and shielding in inner electrons
- bigger atoms are more ready to give up the outer electrons compared to the smaller atoms
- outer electrons of bigger atoms feel less attracted to the nuucleus so they remove more easily
- smaller atoms are more attracted to the nucleus
- increases across a period and decreases down a group
- related to atomic size
- outer electrons feel lesser pull from the nucleus bc of the inner electrons
- repulsion and shielding in inner electrons
- bigger atoms are more ready to give up the outer electrons compared to the smaller atoms
- outer electrons of bigger atoms feel less attracted to the nuucleus so they remove more easily
- smaller atoms are more attracted to the nucleus
13
New cards
electronegativity
- ability of an atom to attract electrons toward itself in a chemical bond
- increases across a period and decreases down a group
- small atomic size/radius = high __________
- increases across a period and decreases down a group
- small atomic size/radius = high __________
14
New cards
electron affinity
- measure if energy is absorbed when an electron is added to a neutral atom to form a negative ion
- most elements have a negative ________ meaning they do not require energy to gain an electron; instead they release energy
- increases across a period and decreases down a group
- small atomic size/radius = high _________
- nucleus' positive charge can accommodate more since there's less shielding and repulsion from inner electrons
- most elements have a negative ________ meaning they do not require energy to gain an electron; instead they release energy
- increases across a period and decreases down a group
- small atomic size/radius = high _________
- nucleus' positive charge can accommodate more since there's less shielding and repulsion from inner electrons
15
New cards
metallic property
- increases down a group
- decreases across a period
- decreases across a period
16
New cards
non-metallic property
- decreases down a group
- increases across a period
- increases across a period
17
New cards
how compounds form
- interaction of electrons (outer) of 1 atom and another atom
- attraction between the nucleus and the electrons of another compound being greater than the repulsion of electron-electron and nucleus-nucleus
- attraction between the nucleus and the electrons of another compound being greater than the repulsion of electron-electron and nucleus-nucleus
18
New cards
chemical bonds
- in any compound formation there are _______ formed
- attractive force that holds 2 atoms together in a more complex unit
- result of interaction between electrons found in the combining atoms
- attractive force that holds 2 atoms together in a more complex unit
- result of interaction between electrons found in the combining atoms
19
New cards
valence electrons
- electrons in outermost electron shell
- only electrons that interact during chemical bond formation
- only electrons that interact during chemical bond formation
20
New cards
Lewis Symbol
chemical symbol of an element surrounded by dots (representation of valence electrons present in atoms of the element)
21
New cards
valence electron configurations of noble gases
certain arrangements of valence electrons are more stable; __________ are most stable bc noble gases have 8 electrons surrounding them
22
New cards
octet rule
formation of chemical bonds aims for electrons/elements to have 8 surrounding electrons
23
New cards
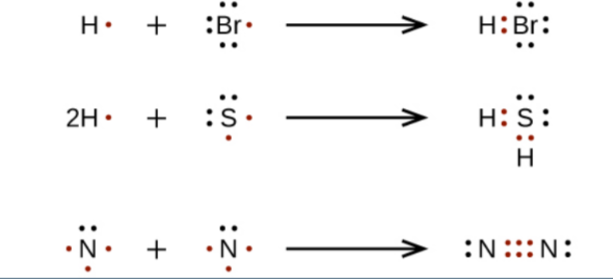
formation of compounds
atoms of elements lose, gain, or share electrons to produce a "noble-gas electron configuration" for each of the atoms involved
24
New cards
ionic bonds
bond formed through the transfer of 1 or more electrons from 1 atom/group of atoms to another atom/group of atoms
25
New cards
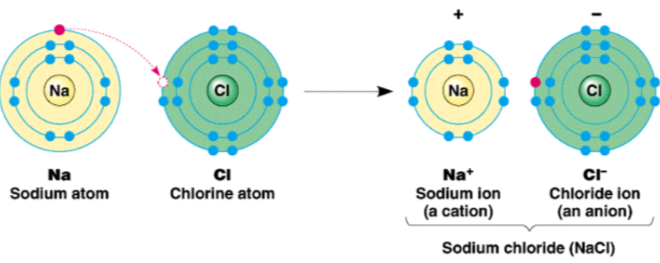
ionic compounds
- always neutral, no net charge
- positive + negative = neutral
- has ionic bonds
- the ratio in which positive and negative ions combine is the ratio that achieves charge neutrality for the resulting compound
- positive + negative = neutral
- has ionic bonds
- the ratio in which positive and negative ions combine is the ratio that achieves charge neutrality for the resulting compound
26
New cards
covalent bonds
chemical bond formed through sharing of 1 or more pairs of electrons between 2 atoms
27
New cards
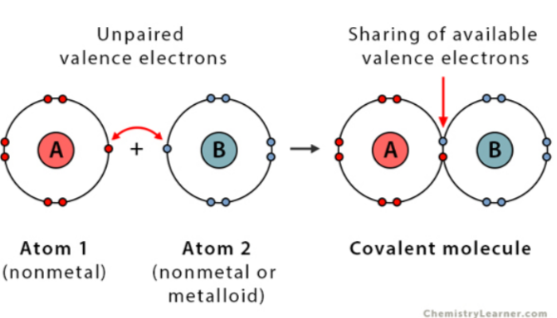
covalent compound
interaction between 2 or more nonmetals (or other atom involved could be metalloid)
28
New cards
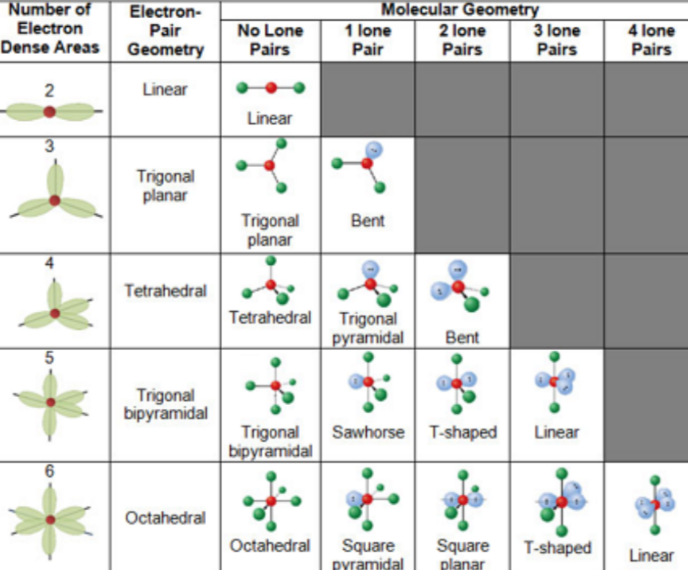
Valence Shell Electron Pair Repulsion Theory (VSPER Theory)
used to predict the shape of compounds
29
New cards
shape of molecule/molecular shape
- determined by its bond angles and repulsions between all the electrons present in the valence/outermost shell of the atom
- predicted by assuming the valence electrons repel each other (bc all of them are negatively charged)
- molecule adopts whichever 3D geometry minimized the repulsion
- predicted by assuming the valence electrons repel each other (bc all of them are negatively charged)
- molecule adopts whichever 3D geometry minimized the repulsion
30
New cards
electron pairs in the valence shell of the central atom
- repel each other and align themselves to minimize repulsion bc repulsion decreases stability of compound
31
New cards
organization/arrangement in compound
- occurs to minimize repulsion
- lessens interactions between electron pairs (specifically lone pairs)
- lessens interactions between electron pairs (specifically lone pairs)
32
New cards
lone pair electrons
- take up more space (than the substituents)
- attracted to 1 nucleus but bond pair is shared by 2 nuclei (covalent bond)
- attracted to 1 nucleus but bond pair is shared by 2 nuclei (covalent bond)
33
New cards
Lewis Structure
using _______, we are able to see which atoms are physically connected to which
34
New cards
how to know molecular shape of compound
- look at # of lone pairs and # of substituents
- assign a shape where the repulsion of electrons is low
- assign a shape where the repulsion of electrons is low
35
New cards
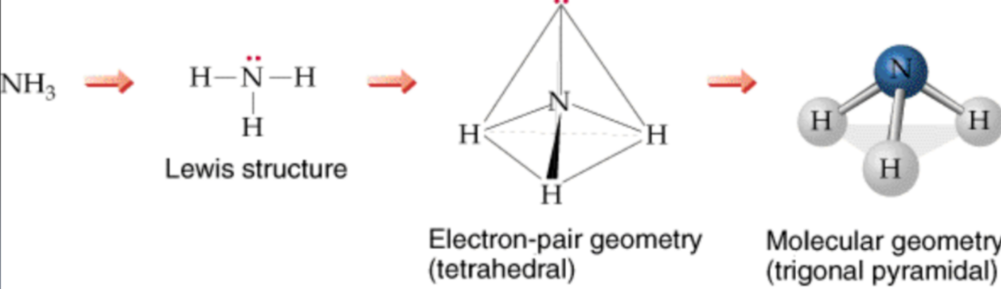
predicting molecular geometrics
1. draw the Lewis Structure
2. count the total number of electron pairs around the central atom
3. arrange the electron pairs such that electron-electron repulsion is at a minimum
2. count the total number of electron pairs around the central atom
3. arrange the electron pairs such that electron-electron repulsion is at a minimum
36
New cards
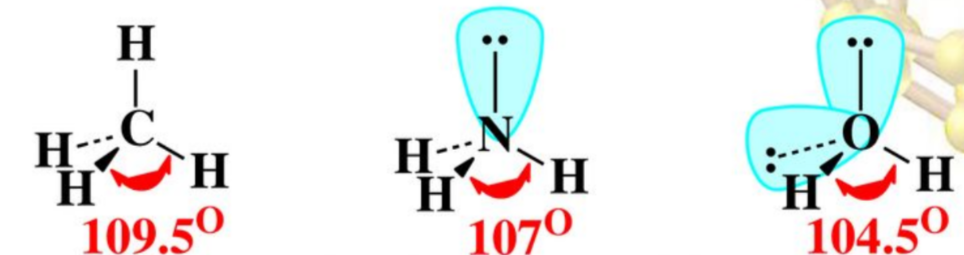
effect of nonbonding electrons and multiple bonds on bond angles
- lone pairs repel more than the bond electrons bc the latter are attracted by 2 nuclei
- bond angle decreases as the number of lone pairs increase
- bond angle decreases as the number of lone pairs increase
37
New cards
polarity
- means there are dipoles (a positive and negative end)
- arises from the difference in electronegativity between 2 bonded atoms
- arises from the difference in electronegativity between 2 bonded atoms
38
New cards
dipole moments
- occurs when atom is electronegative, resulting in a tendency to pull bond electrons towards itself
- once formed, there is partially negative and partially positive ends, hence polar molecules and non-polar molecules
- do not add up to 0 or do not cancel out in polar molecules
- once formed, there is partially negative and partially positive ends, hence polar molecules and non-polar molecules
- do not add up to 0 or do not cancel out in polar molecules
39
New cards
quantum mechanical model of the atom
- most accurate and accepted model of the atom
- derives from the Schrodinger wave equation and deals with probabilities
- electrons do not exist as tiny points inside the atom but instead surround the nucleus in a form resembling a cloud
- derives from the Schrodinger wave equation and deals with probabilities
- electrons do not exist as tiny points inside the atom but instead surround the nucleus in a form resembling a cloud
40
New cards
probabilities
electrons' position (not the specific position/space of electrons' location)
41
New cards
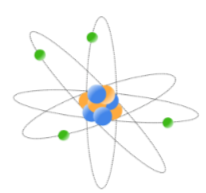
electron orbit model
originally, electrons were thought to orbit around the nucleus in defined paths
42
New cards

electron cloud model
- it was discovered that electrons move in waves in a defined space called an electron cloud
- can't pinpoint where an electron is at a specific time
- can't pinpoint where an electron is at a specific time
43
New cards
Heisenberg Uncertainty Principle
- position and momentum of an electron cannot be determined simultaneously w/ absolute accuracy
- if you know the position of an electron you cannot know the momentum of that electron, and vice versa
- if you know the position of an electron you cannot know the momentum of that electron, and vice versa
44
New cards
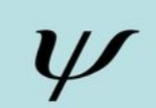
wave function
- helps comprehend the state of a quantum mechanical system
- can be real or imaginary
- no meaning can be assigned to it as it is
- originates from Schrodinger's wave equation
- can be real or imaginary
- no meaning can be assigned to it as it is
- originates from Schrodinger's wave equation
45
New cards
wave function equation
- compiles the values/variables in order for us to know location or momentum of electron
- cannot really tell us anything about the electron
- cannot really tell us anything about the electron
46
New cards
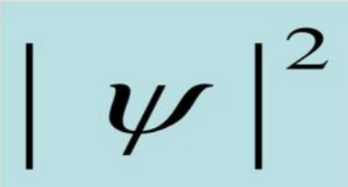
square of the function
- only quantity that has some meaning according to Max Born
- indicates the probability of finding an electron near a particular point in space at a particular time
- indicates the probability of finding an electron near a particular point in space at a particular time
47
New cards
probability density
we cannot entirely say where the location is of an electron
48
New cards
probability distribution
- intensity of color is used to indicate the probability value near a given point in space
- used after subjecting a specific atom to know where the electron is
- if color in specific area is intense, electrons are most likely there
- color intensity tells us electrons are dense in specific area
- used after subjecting a specific atom to know where the electron is
- if color in specific area is intense, electrons are most likely there
- color intensity tells us electrons are dense in specific area
49
New cards
Principal quantum number (n)
- distance from nucleus
- size and energy of orbital
- how close/far is the orbital from the nucleus
- is the number itself in electron configuration
- based on numbers, 1 is closest to nucleus
- size and energy of orbital
- how close/far is the orbital from the nucleus
- is the number itself in electron configuration
- based on numbers, 1 is closest to nucleus
50
New cards
Angular momentum quantum number (l)
- shape of orbital
- shape of atomic orbitals (sometimes called a subshell)
- are the letters in electron configuration (s, p, d, f, g)
-> s = spherical
-> p = dumbbell
- shape of atomic orbitals (sometimes called a subshell)
- are the letters in electron configuration (s, p, d, f, g)
-> s = spherical
-> p = dumbbell
51
New cards
Magnetic quantum number (m)
- orientation in space
- axis/portion of space that electrons occupy
- orientation of orbital in space relative to the other orbitals in the atom
-> location/area that electrons occupy
- acquired based on the value of the angular momentum number
-> letters (s, p, d, f) have specific numerical values
-> s = 0, p = 1, d = 2, f = 3
- possible values range from negative angular momentum quantum number to positive angular momentum quantum number
- axis/portion of space that electrons occupy
- orientation of orbital in space relative to the other orbitals in the atom
-> location/area that electrons occupy
- acquired based on the value of the angular momentum number
-> letters (s, p, d, f) have specific numerical values
-> s = 0, p = 1, d = 2, f = 3
- possible values range from negative angular momentum quantum number to positive angular momentum quantum number
52
New cards
electron spin (s)
either positive or negative spin
53
New cards
orientation in space
based on 3 axis (x, y, z)
54
New cards
orbitals
show where the electrons are
55
New cards
molecular geometry of water
- molecule is polar bc dipole moments is pointed upwards
- electronegative oxygen pulls electrons towards itself
- electronegative oxygen pulls electrons towards itself
56
New cards
water
- universal solvent (dissolves more substances than any other liquid)
- is bent and is a polar molecule due to dipole moment
- follows the "like dissolves like" general rule in terms of solubility (p molecules dissolves p molecules, np molecules dissolves np molecules)
- partial changes in molecule attract parts of other polar molecules to dissolve with them
- is bent and is a polar molecule due to dipole moment
- follows the "like dissolves like" general rule in terms of solubility (p molecules dissolves p molecules, np molecules dissolves np molecules)
- partial changes in molecule attract parts of other polar molecules to dissolve with them
57
New cards
Intermolecular Forces of Attraction (IMFA)
- attractive forces between molecules
- called van der Waals forces, named after Dutch scientists Johann van der Waals
- called van der Waals forces, named after Dutch scientists Johann van der Waals
58
New cards
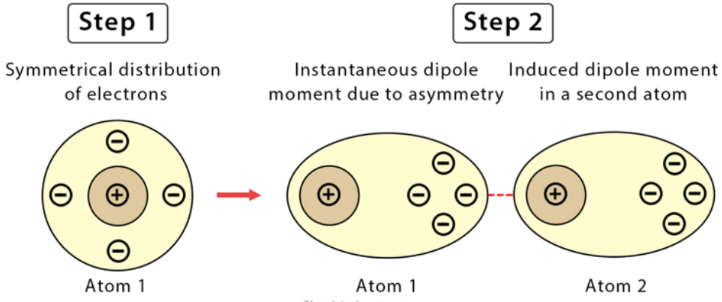
London Dispersion Forces (LDF)
- present in all molecules
- due to the fluctuations in the electron distribution within atoms or molecules
- forces are particularly weak
- due to the fluctuations in the electron distribution within atoms or molecules
- forces are particularly weak
59
New cards
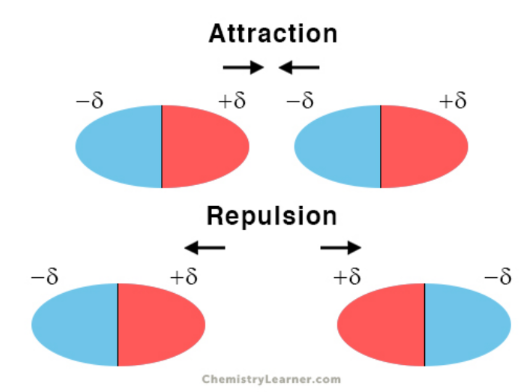
Dipole-Dipole Forces
- attractive forces between polar molecules
- isn't just from instantaneous dipole
- induced by the presence of electronegative atoms
- due to electrical interactions among dipoles on neighboring molecules
- partially positive pole will attract partially negative pole
- repulsion occurs if atoms that are positive-positive poles or negative-negative poles are directed towards each other
- moderately strong bc dipole moment of polar molecules are not instantaneous
- isn't just from instantaneous dipole
- induced by the presence of electronegative atoms
- due to electrical interactions among dipoles on neighboring molecules
- partially positive pole will attract partially negative pole
- repulsion occurs if atoms that are positive-positive poles or negative-negative poles are directed towards each other
- moderately strong bc dipole moment of polar molecules are not instantaneous
60
New cards
Hydrogen Bonding
- special type of dipole-dipole force
- attractive force between a hydrogen atom of 1 molecule and a highly electronegative atom (O, N, F) of another molecule
- strongest type
- H will attract an electronegative atom to another molecule and it can either be:
-> intermolecular H bonding = between several molecules
-> intramolecular H bonding = within 1 molecule
- attractive force between a hydrogen atom of 1 molecule and a highly electronegative atom (O, N, F) of another molecule
- strongest type
- H will attract an electronegative atom to another molecule and it can either be:
-> intermolecular H bonding = between several molecules
-> intramolecular H bonding = within 1 molecule
61
New cards
application of IMFA
- when molecules have strong intermolecular forces of attraction, they are packed close together; they may also have a relatively high boiling point and/or melting point since it requires a higher amount of energy to break the bond
-> they often exist as a condensed phase (solid or liquid) at room temperature
- when molecules have weak intermolecular forces of attraction, they are far apart from each other, they often exist as gas at room temperature
-> they often exist as a condensed phase (solid or liquid) at room temperature
- when molecules have weak intermolecular forces of attraction, they are far apart from each other, they often exist as gas at room temperature
62
New cards
solubility
- ability of substance to dissolve in a given amount of solvent at a specified temperature
- when the solute and solvent both exhibit the same intermolecular forces of attraction, they form a solution
- when the solute and solvent both exhibit the same intermolecular forces of attraction, they form a solution
63
New cards
carbohydrates
- polyhydroxy aldehydes and ketones
-> poly = many; hydroxy = OH portion
-> aldehydes and ketones = has a carbonyl group in the molecule
- composed of carbon, hydrogen, and oxygen
-> poly = many; hydroxy = OH portion
-> aldehydes and ketones = has a carbonyl group in the molecule
- composed of carbon, hydrogen, and oxygen
64
New cards
monosaccharides
- simple sugars with 3-7 carbons with a carbonyl group at either terminal C or the C adjacent to it
65
New cards
aldoses
aldehyde group at C1
66
New cards
ketoses
ketone group at C2
67
New cards
disaccharides
- contain 2 monosaccharides linked by a glycosidic bond
- formation proceeds via condensation reaction aka dehydration reaction
-> water molecule is abstracted/removed from the glycosidic bond
- formation proceeds via condensation reaction aka dehydration reaction
-> water molecule is abstracted/removed from the glycosidic bond
68
New cards
polysaccharides
more than 2 monosaccharides
69
New cards
cellulose and amylose
- straight chain
- straight linkage of glucose units between carbon 1 and 4 of another glucose unit
- straight linkage of glucose units between carbon 1 and 4 of another glucose unit
70
New cards
amylopectin and glycogen
- straight chain linkages between carbon 1 and 4 of glucose units
- when it branches out, carbon 1 is connected/bonded to carbon 6 of a glucose unit
- when it branches out, carbon 1 is connected/bonded to carbon 6 of a glucose unit
71
New cards
Molisch test
test = for all sugars (steps: dehydration, condensation, and oxidation)
reagent(s) = 1-napthol, ethanol, H2SO4
positive indication = purple interface
- if this test is performed and you see a purple interface, then there is most likely a carbohydrate in your sample
reagent(s) = 1-napthol, ethanol, H2SO4
positive indication = purple interface
- if this test is performed and you see a purple interface, then there is most likely a carbohydrate in your sample
72
New cards
Benedict's test
test = for reducing sugars
reagent(s) = CuSO4, Na2CO3, sodium citrate
positive indication = brick red-precipitate (if high concentration, green, yellow, or orange precipitates)
reagent(s) = CuSO4, Na2CO3, sodium citrate
positive indication = brick red-precipitate (if high concentration, green, yellow, or orange precipitates)
73
New cards
Fehling's test
test = for reducing sugars
reagent(s) = CuSO4, Na2CO3, sodium tartate
positive indication = brick-red precipitate (same as Benedict's Test)
reagent(s) = CuSO4, Na2CO3, sodium tartate
positive indication = brick-red precipitate (same as Benedict's Test)
74
New cards
Osazone formation
test = for identification
reagent(s) = phenylhydrazine
positive indication = yellow crystals
- after formation of crystals, you will subject it to melting point and compare to literature values to know the identity of your carbohydrate
reagent(s) = phenylhydrazine
positive indication = yellow crystals
- after formation of crystals, you will subject it to melting point and compare to literature values to know the identity of your carbohydrate
75
New cards
functions of carbohydrates
- to store and provide energy
- when glucose is further broken down, the energy released by breaking its chemical bonds are sued/stored by the body in the form of glycogen
- when glucose is further broken down, the energy released by breaking its chemical bonds are sued/stored by the body in the form of glycogen
76
New cards
proteins
macromolecules comprised of 1 or more long chains of amino acid residues joined together by peptide bonds
77
New cards
amino acids
basic unit of proteins
78
New cards
oligopeptides
contain up to about 50 amino acid residues
79
New cards
polypeptides
contain more than 50 amino acid residues
80
New cards
2 ends of a peptide
N-terminal and C-terminal
81
New cards
N-terminal
end with free amino group
82
New cards
C-terminal
end with carboxyl group
83
New cards
peptide bond
- bond between amino acid residues
- formation proceeds via dehydration reaction
- lone pair of nitrogen attacks the partially positive carbonyl carbon; a water molecule is abstracted
- formation proceeds via dehydration reaction
- lone pair of nitrogen attacks the partially positive carbonyl carbon; a water molecule is abstracted
84
New cards
primary structure
- the sequence of amino acid comprising the polypeptide chain
- peptide bonds
- peptide bonds
85
New cards
secondary structure
- the folding of the polypeptide
- hydrogen bonds between the amino and carboxyl groups of non-adjacent amino acids
- hydrogen bonds between the amino and carboxyl groups of non-adjacent amino acids
86
New cards
tertiary structure
- the 3D shape of the polypeptide
- interactions between the side chains, hydrogen bonding, disulfide bridges, electrostatic and hydrophobic interactions
- interactions between the side chains, hydrogen bonding, disulfide bridges, electrostatic and hydrophobic interactions
87
New cards
quaternary structure
- inter-chain interactions; formation of subunits
- hydrogen bonds and van der Waals forces between non-polar side chains
- hydrogen bonds and van der Waals forces between non-polar side chains
88
New cards
functions of proteins
- they can act as catalysts and hasten chemical reactions; they can transport substances and provide structural support
- many of them function as enzymes (molecules that catalyze or speed up chemical reactions in the body)
- many of them function as enzymes (molecules that catalyze or speed up chemical reactions in the body)
89
New cards
lipids
- consist of a heterogenous group of compounds
- insoluble in water and soluble in non-polar solvents due to the large # of C-C and C-H
- defined on the basis of a physical property and not by presence of functional group
- insoluble in water and soluble in non-polar solvents due to the large # of C-C and C-H
- defined on the basis of a physical property and not by presence of functional group
90
New cards
biological functions of lipids
- store chemical energy in the body
- insulate vital organs
- covering of nerve fibers
- components of cell membrane
- chemical messengers (i.e. hormones)
- insulate vital organs
- covering of nerve fibers
- components of cell membrane
- chemical messengers (i.e. hormones)
91
New cards
saponifiable lipids/hydrolyzable lipids
- can undergo hydrolysis/can hydrolize
- lipids that are converted into smaller molecules by hydrolysis
- contains an ester unit
- lipids that are converted into smaller molecules by hydrolysis
- contains an ester unit
92
New cards
tricylglycerols/fats/oils
- esters of fatty acids with glycerol
- main storage form of fatty acids
- contains glycerol and 3 fatty acid chains
- fatty acid attached to glycerol can either be saturated or unsaturated
- main storage form of fatty acids
- contains glycerol and 3 fatty acid chains
- fatty acid attached to glycerol can either be saturated or unsaturated
93
New cards
waxes
esters of fatty acids with glycerol
94
New cards
phospholipids
- contains phosphoric acid residue in addition to an alcohol and fatty acids
- major component of cell membranes
- similar to triglyceride but 1 fatty acid is replaced by phosphate group (polar head)
- forms a lipid bilayer; hydrophobic tails are sandwiched between hydrophilic heads
- lipid bilayer acts as barrier to the passage of molecules and ions into and out of the cell
- various protein molecules are embedded in and through the lipid bilayer
- major component of cell membranes
- similar to triglyceride but 1 fatty acid is replaced by phosphate group (polar head)
- forms a lipid bilayer; hydrophobic tails are sandwiched between hydrophilic heads
- lipid bilayer acts as barrier to the passage of molecules and ions into and out of the cell
- various protein molecules are embedded in and through the lipid bilayer
95
New cards
glycerophospholipids
glycerol as a source of alcohol group
96
New cards
sphingolipid
sphingosine as source of alcohol group
97
New cards
glycolipids
contain carbohydrate in addition to sphingosine and fatty acid
98
New cards
non-saponifiable or non-hydrolyzable lipids
- lipids that cannot be broken down into smaller components by aqueous hydrolysis
- examples are steroids and terpenes
- examples are steroids and terpenes
99
New cards
nucleic acids
- polymers also called polynucleotide
-> nucleotide = consists of a nitrogenous base, a pentose sugar, and one or more phosphate groups
-> nucleoside = nucleotide without the phosphate group
-> nucleotide = consists of a nitrogenous base, a pentose sugar, and one or more phosphate groups
-> nucleoside = nucleotide without the phosphate group
100
New cards
Phosphodiester linkages
what joins nucleotides together between the 5' and 3' OH group of two separate nucleotides