Chapter 10 Properties of Gases
1/17
Earn XP
Description and Tags
Chapter 10
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Gas
A gas is a substance that has no well defined boundaries but diffuses readily to fill any container in which it is placed in.
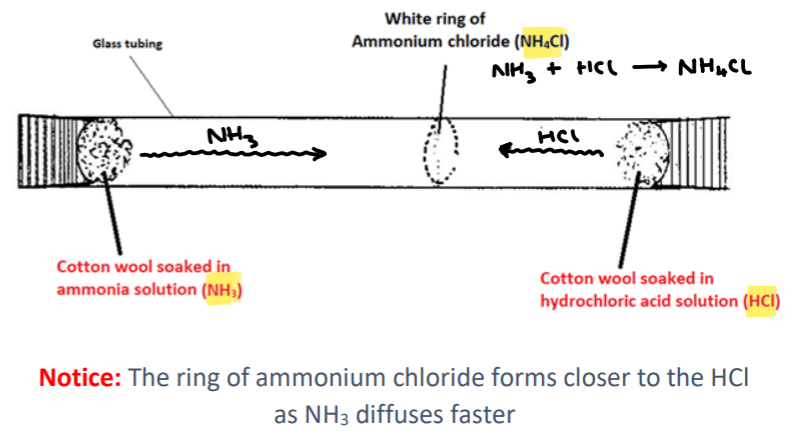
What is diffusion?
• Diffusion is the movement of a substance from an area of high concentration to an area of low concentration.
Note: Diffusion applies to liquids and gases as the particles can move
Properties of gases
no definite shape or volume
can be compressed
definite mass
Temperature
Temperature is the degree of hotness of an object.
pressure
The pressure of gas is the force that the gas exerts on a unite area of its container
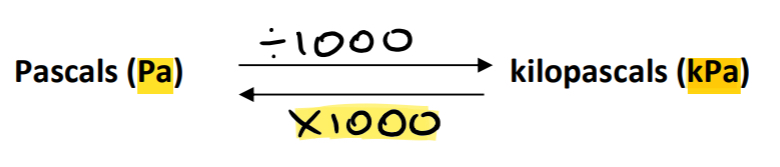
Volume
the amount of space a gas takes up
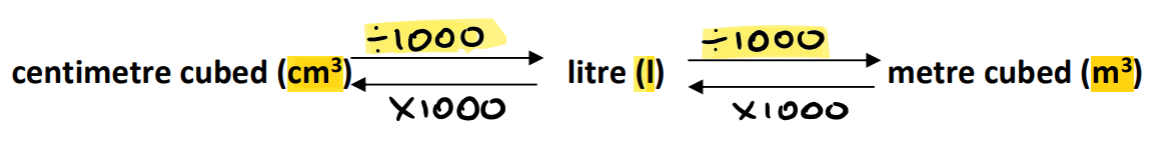
Boyle’s Law
Boyle's Law states that at constant temperature, the volume of a fixed mass of gas is inversely proportional to its pressure. PV=k
P1V1 = P2V2
Charle’s Law
Charles' Law states that, at constant pressure, the volume of a fixed mass of gas is directly proportional to its temperature measured on the Kelvin scale.
V1/T1 = V2/T2
The combined gas law
(P1V1)/T1 = (P2V2)/T2
Gay- Lussac's Law of Combining Volumes
In a reaction between gases, the volumes of the reacting gases and the volumes of any gaseous products are in the ratio of small whole numbers provided the volumes are measured at the same temperature and pressure.
Avagadro's Law
Avagadro's Law states that equal volumes of gases contain equal numbers of molecules under the same conditions of temperature and pressure.
The Kinetic Theory of Gases (movement)
1. Gases are made of particles that are in a continuous and rapid, random movement and collide with each other and the walls of its container.
2. There are no attractive or repulsive forces between molecules of gas.
3. Gas molecules are so small and so widely separated that the actual volume of all the molecules is negligible compared with the space that they occupy.
4. Collisions between particles are perfectly elastic, i.e. there is no loss of kinetic energy in these collisions although there may be a transfer of energy.
5. The average kinetic energy of the particles in a sample of gas is proportional to the temperature measured on the Kelvin scale.
What is an ideal gas?
• An ideal gas is one that perfectly obeys all the gas laws under all conditions of temperature of pressure
Note: However, ideal gases DO NOT exist
Limitations to the Kinetic Theory of Gases
There are forces of attraction between gas molecules e.g. Van Der Waals, dipole- dipole
It is not valid to say that the total volume of gases is always negligible compared with the space that they occupy. Under high pressure, molecules are crowded closer together.
The kinetic theory of gases only holds true for an ideal gas - no such gas exists.
Condition where real gas comes close to being an ideal gas
At low pressure when the molecules are widely spaced.
High pressure forces molecules closer together thus making the particles compared to the volume not negligible
and increases the chances of attraction or repulsion between particles.
At high temperatures when the molecules are moving rapidly, preventing the forces between molecules from operating.
Low temperatures cause the particles to move slowly thus increasing their chances of attraction or repulsion
occurring.
The Equation of State for an Ideal Gas
PV=nRT
P= pressure (pascals)
V = Volume (m3)
n = number of moles
R= Universal gas constant =8.31 Jmol-1 K-1
T = Temperature in Kelvin
Which of the noble gases would you expect to behave most like an ideal gas? Justify your answer.
• Helium
• Ideal gases have no attractive or repulsive forces between the molecules
• Helium is the noble gas with the smallest mass – and therefore has the weakest van der waals forces (The strength of intermolecular forces decreases as mass decreases)
to measure the relative molecular mass of a volatile liquid
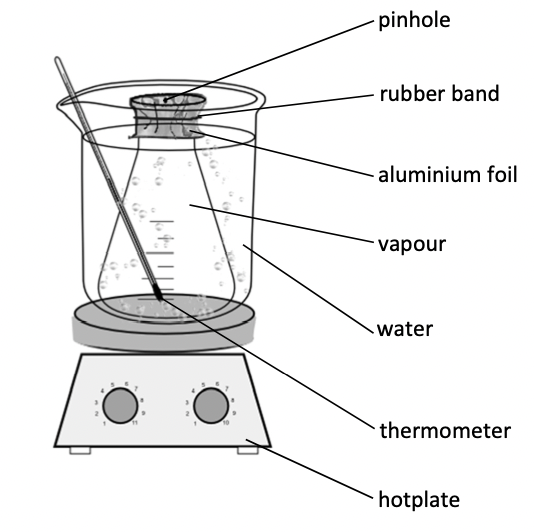
Get the mass of a clean, dry conical flask including the tinfoil and rubber band.
Place approx. 10 cm' of propanone into the conical flask and put the tin foil with a rubber band around the top of the conical flask.
Put a pin hole in tin foil to ensure pressure inside the conical flask is the same outside the conical flask.
Place conical flask in a water bath and heat until liquid has vaporised.
Remove the flask from the water bath and dry. Allow to cool.
Reweigh the conical flask, tinfoil, band and vapourised liquid and get the difference - this is the mass of the volatile liquid.
Get the volume of the conical flask by filling it entirely with water and pouring it into a graduated cylinder.
Calculate Mr.