alkenes
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
84 Terms
What are alkenes and their general formula?
Alkenes are unsaturated hydrocarbons because they contain a C=C double bond and so the general formula CnH2n. They have no rings
What is a cycloalkene?
A hydrocarbon which contains carbon-carbon double bonds, and also contains a ring is called a cycloalkene
naming simple alkenes
The position number should be the lowest number carbon involved in the double bond
what is this molecule called
There is a double bond so it is an alkene therefore it ends with an ‘ene’
There are 4 carbon in the longest chain so it starts with the prefix ‘but’
The double bond is on the 1st carbon so we add the position number after the prefix and before the ending ‘ene’.
Why don’t ethene and propene need a position number for the double bond?
For both ethene and propene we don't need to add the position number because there is only 1 position for the double bond to go
position numbers for branched alkenes
Position number - name of side chain dependent on number of carbon in side chain- ‘yl - name of longest chain of carbons (dependent on number of carbon in long chain) - position number of double bond - ‘ene’
How do you number the main chain of an alkene?
we give HIGHER priority to giving the double bond the lower number than to giving a methyl group/side chain a lower number.
How do you choose the main chain in a branched alkene?
A branched alkenes main chain is the longest chain which INCLUDES the double bond
Properties of alkenes
Alkenes have a high electron density around the C=C double bond. Being composed only of C=C, C-C, and C-H bonds, they are nonpolar molecules and experience only van der Waals forces. As the carbon chain length increases, the number of electrons increases, strengthening van der Waals forces and raising melting and boiling points. Alkenes are also highly insoluble in water.
Explain why E/Z isomerism occurs in certain alkenes
Why do some alkenes have stereoisomers/ what are stereoisiomers
Because there is restricted rotation around a double bond some alkenes have two stereoisomers Stereoisomers are molecules with the same structural formula but different spatial arrangements
What is the difference between structural isomers and stereoisomers?
Structural isomers have the same molecular formula but different structural formulas.
In contrast stereoisomers have the same molecular formula, the same structural formula but different spatial arrangements.
Why are some molecules not considered isomers?
The molecules on the right are not isomers at all because they contain a single bond which means the carbon can freely rotate such that they look identical
substituents
To find how many substituents an alkene has we have we count the substituents bonded to the carbons involved in the doble bond
A substituent is an atom or group of atoms which could be replaced by a hydrogen atom.
how many substituents does this alkene have
This alkene has 4 substituents
z isomers and e isomers
When a molecule has two substituents on the same side of a double bond, we label it the Z isomer. ‘Zee zame zide’
When a molecule has two substituents on opposite sides of a double bond, we label it the E isomer.
How do we indicate the type of alkene isomer in its name?
Once we have decided which isomer it is we add the capital letter Z or E to the beginning of the molecules name
E and Z isomerism with 3 substituents
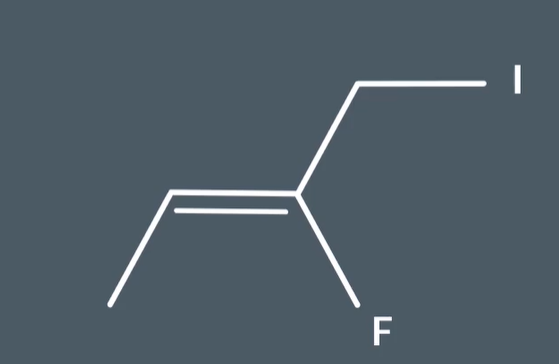
Is this an e or z isomer
Look at the 2 atoms bonded directly to cabron in the double bond which is carbon (NOT IODINE) and fluorine
Compare atomic numbers
Fluorine has a higher atomic number to carbon so it takes priority
Look at the double bond acid and determine whether it is an E or Z isomer using priority substituent
Of the 2 substituents on the right F substituent takes priority and it is on the same side as the substituent on the left so it is a Z isomer
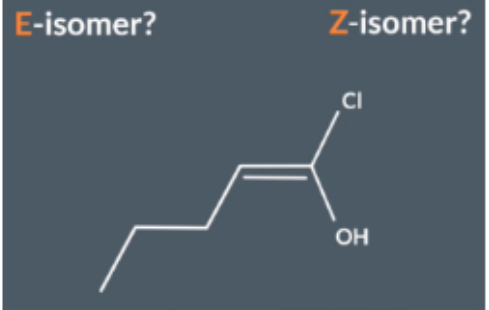
Is this an E or Z isomer
Look at 2 atoms bonded to the end of the C=C bond
Cl and O NOT OH
Compare atomic numbers
Cl (17) has a higher atomic number to oxygen ( 9) Cl it takes priority
Look at the double bond axis and determine whether it is an E or Z isomer using priority substituent
Cl is on the opposite side of the double bond axis so this is a E isomer
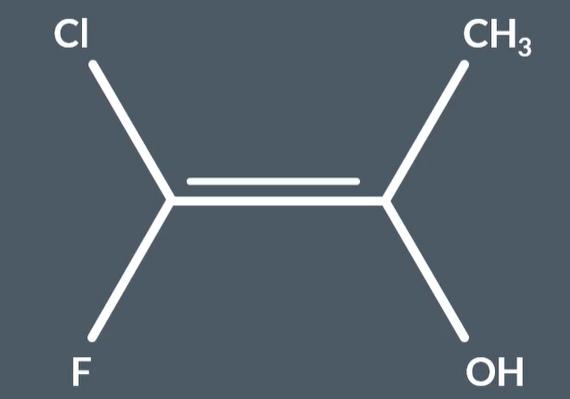
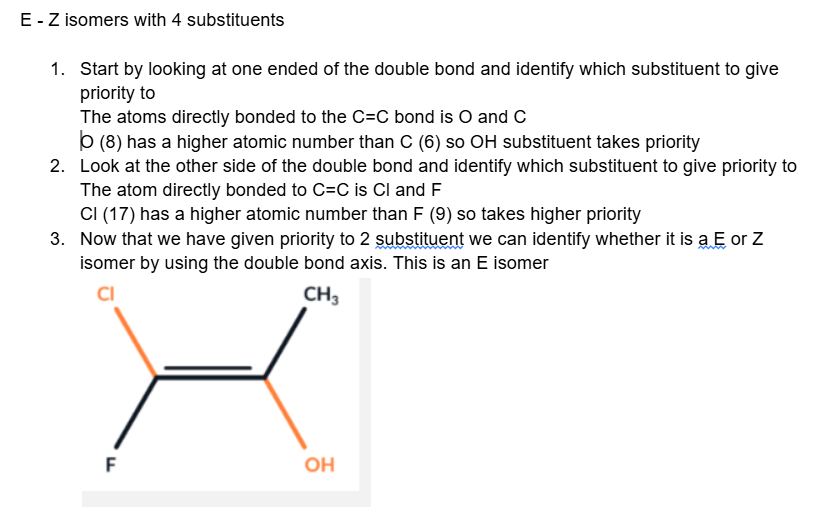
E - Z isomers with 4 substituents
what happens if there is a tie in atomic number
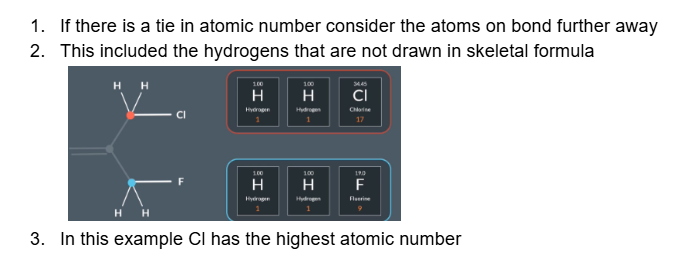
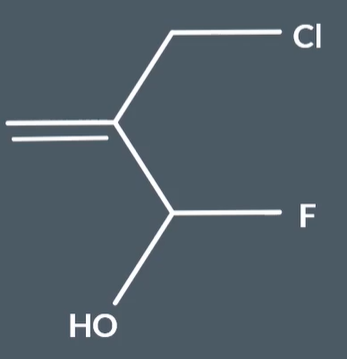
What is the highest priority substituent
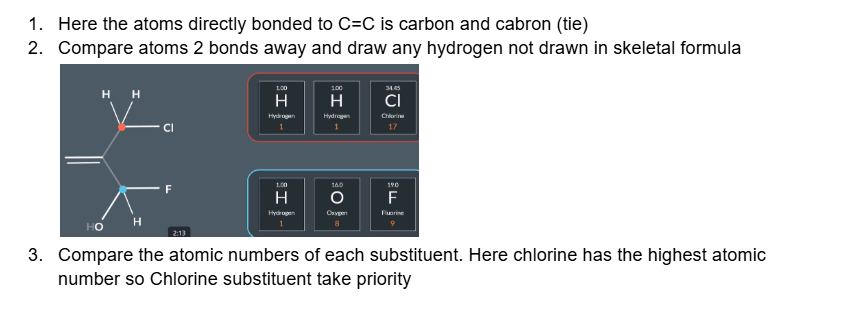
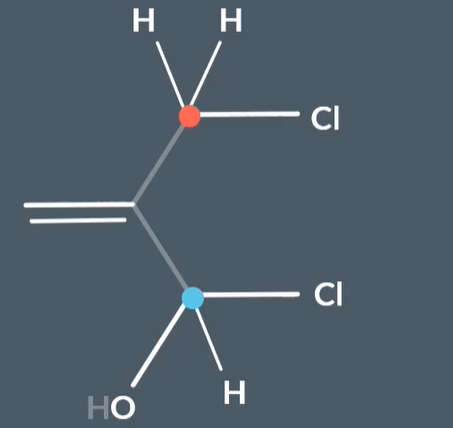
Which substituent has higher priority here

Naming alkene isomers using CIP notation
If the alkene coud have stereoisomers we assign priority to one of the substituents at each end of the double bond and add an E or Z to the start of the name. (CIP notation)

Do we use CIP notation for this molecule
In this molecule the two substituents on the right hand side are identical. SO this molecule does not show stereoisomerism and we don't use CIP notation in its name
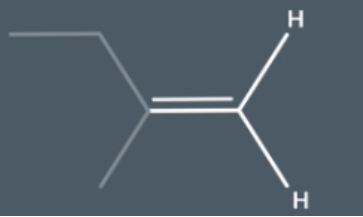
do we sue E-Z notation on this moleucle
In this molecule there aren't any substituents on the right hand side so we don't use CIP notation in its name instead we call it 2 methylbut-1-ene and we don't add E or Z to the start of its name
How to test for alkenes
Bromine solution turns reddish brown in the presence of carbon-carbon double bonds (C=C) but not single bonds
Adding bromine to an alkene trunks Reddish brown. Causes an electrophilic addition reaction
additon reactions
converting an alkene into a halogenoalkane in the presense of Br2 or HX
What is an addition reaction?
Reactions in which two molecules combine to form one molecule are called addition reactions
What intermediate is formed during alkene addition reactions?
In addition reactions, alkenes form intermediates, before they form the final product. These intermediates are called carbocations because they are cations that contain positively charged carbon atoms
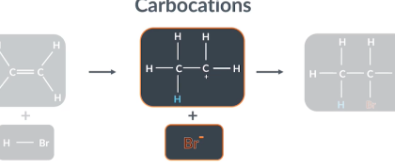
There are two factors that make it possible for a carbon cation to form
Polar bond (H-Br is polar)
Double bond have high electron density
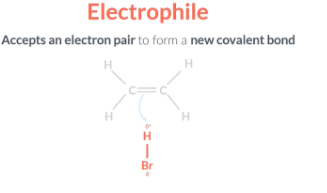
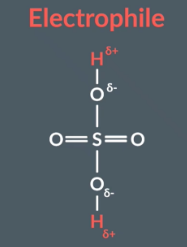
Electrophilic addition
A substance that accepts an electron pair to form a new covalent bond is called a electrophile. In the example above the H-Br acts as an electrophile because it accpets two electrons form the double bond. Therefore we call it electrophilic addition
When drawing addition reactions we draw
Starting reactants - step 1
Intermediate - step 2
Final product - step 3
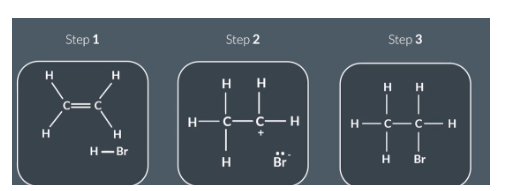
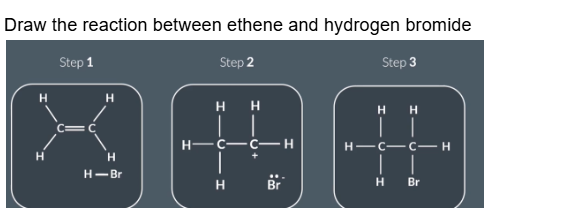
Draw the reaction between ethene and hydrogen bromide
Reactants
Bond forms between C and H
H-BR bond breaks
Intermediate
Bond forming:
Lone pair on Br- forms bond with partial positive C
Final products
No curly arrows
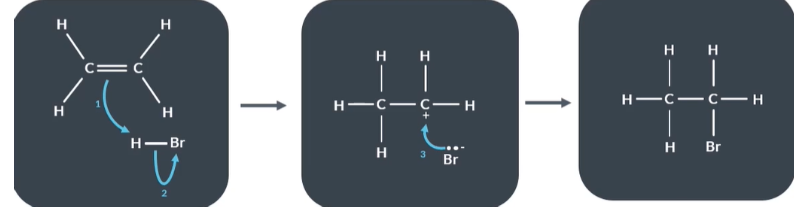
How to draw curly arrow mechanism
Draw the reactants, intermediates and final products
Deduce which bonds are breaking and which are forming
Draw the curly arrows
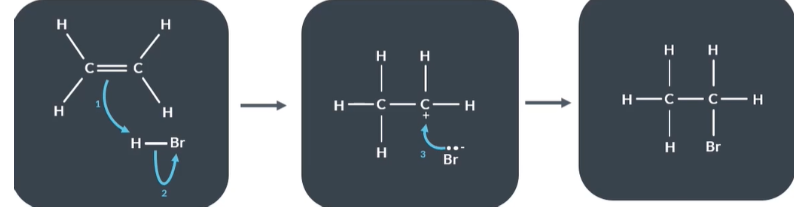
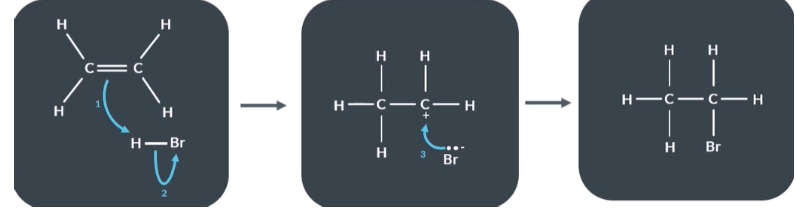
Using these curly arrow mechanism describe what happens in a reaction between ethene and hydrogen bromide
Reactants
Electron pair in the pi bond (C=C) has formed a new bond between C and H
The H Br bond breaks and Bromine keeps the 2 electrons that were shared between H and Br
Intermediate
Bromide ions supply the electron pair which form the bond between bromine and cabron .
Ethene and hydrogen bromide reaction:
Once the carbocation is formed…
the positively-charged carbon atom attracts the negatively-charged bromide ion.
The bromide ion supplies 2 electrons
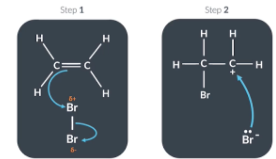
The electrophilic addition reaction between ethene and bromine is possible because
When the bromine molecule approaches the electron dense C=C bond, momentary partial charges occur, allowing bromine to act as an electrophile.
Can be explained by Van der Waals
The movement of electrons cause a temporary imbalance in charge with one side being partially positive and one side being partially negative. Temporary dipole can induce dipoles in nearby molecules. This causes an electrostatic attraction between partially positive side of one molecule and negative side of another molecule.
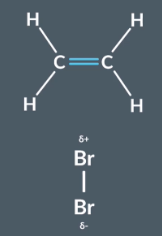
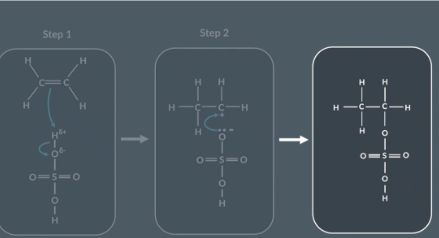
Draw sulfuric acid
Drawing the reaction between ethene and sulfuric acid
Reactants
Bond forms between C and H
H-O bond breaks
Intermediate
Bond forming:
Lone pair on O forms bond with partial positive C
Final products
No curly arrows
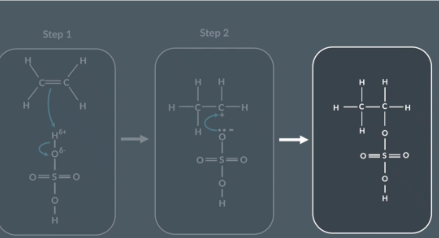
What is the product when ethene reacts with H2SO4
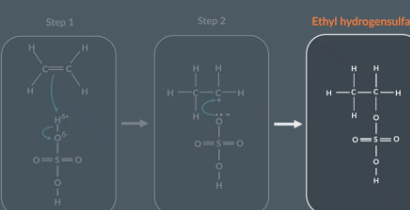
Using these curly arrow mechanism describe what happens in a reaction between ethene and H2SO4
Reactants
Electron pair in the pi bond (C=C) has formed a new bond between C and O
The O H bond breaks and O keeps the 2 electrons that were shared between H and Br
Intermediate
Oxygen atom supply the electron pair which forms the bond between oxygen and carbon
Ethene and Sulfuric acid reaction
Once the carbocation is formed…
the positively-charged carbon atom attracts the negatively-charged oxygen
The oxygen supplies 2 electrons
Why are new bond such as H-Br more likely to form on the carbon that is attached to more hydrogens?
This explain why A new bond such as a (H-Br) bond is more likely to form on the carbon that is attached to more hydrogens (2- bromopropane
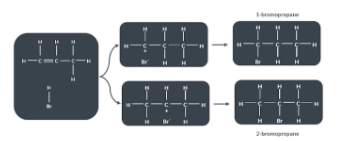
What determines the major and minor products in the addition reaction of unsymmetrical alkenes?
When unsymmetrical alkenes take part in an addition reaction more than one product is possible. A new bond such as a (H-Br) bond is more likely to form on the carbon that is attached to more hydrogens/ alkyl gorupps (CnH2n+1) (2- bromopropane) . We label the two possible product of unsymmetrical alkenes as the major product and the minor product depending on which carbon the molecule is attached to.
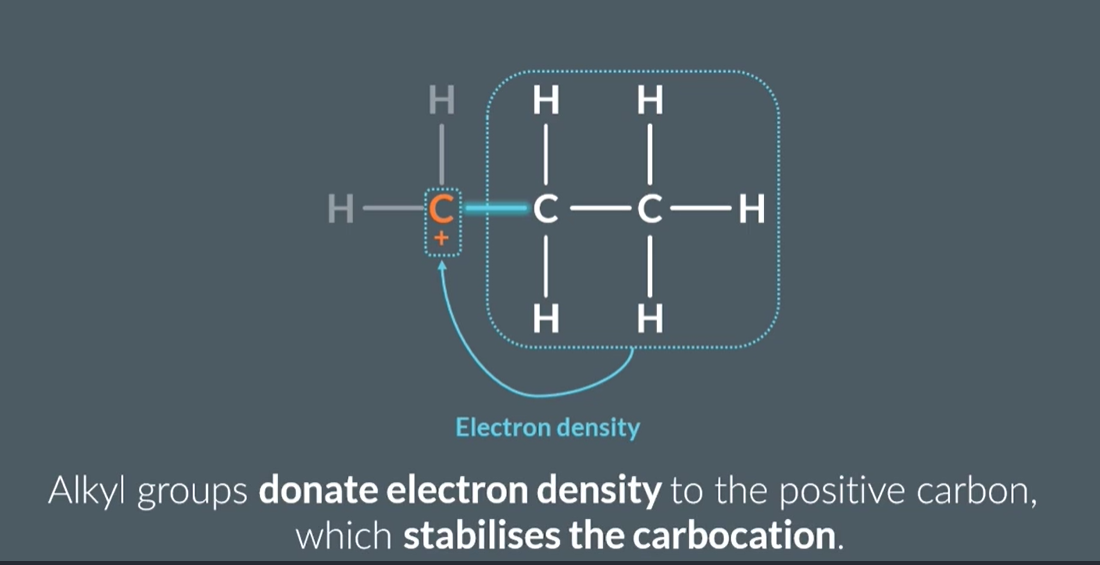
How does the stability of primary, secondary, and tertiary carbocations compare?
Tertiary carbocations are the most stable (due to three alkyl groups donating electron density).
Secondary carbocations are less stable than tertiary but more stable than primary.
Primary carbocations are the least stable (with only one alkyl group donating electron density).
What are the differences between primary, secondary, and tertiary carbocations?
Primary carbocation: Positively charged carbon bonded to 1 alkyl group.
Secondary carbocation: Positively charged carbon bonded to 2 alkyl groups.
Tertiary carbocation: Positively charged carbon bonded to 3 alkyl groups.
Why are tertiary carbocations more stable than primary carbocations, and how does this affect the major and minor products?
Tertiary carbocations are the most stable and primary carbocations are the least stable. This explains why major and minor products look the way they do (minor will probably have a primary carbocation)
What happens during addition polymerization of molecules with double bonds?
Under certain conditions, molecules with double bonds undergo addition polymerization, where each loses its double bond and bonds together to form a long macromolecule called an addition polymer. This process produces no by-products, as all reactants combine directly.
What are monomers
The molecules which are added together in a polymerisation reaction are called monomers
How do you name a polymer according to IUPAC convention?
]
The molecules which are added together in a polymerisation reaction are called monomers. According to the IUPAC convention to name a polymer we:
Take the name of the monomer and put it into brackets
Add a prefix ‘poly’ before it
E.g chloroethene is poly(chloroethene)
Properties of addition polymers
strong because their long chains create strong intermolecular forces.
inert due to their backbone of non-polar carbon-carbon bonds, which very few chemical compounds can break.
Ideal for food containers (don’t corrode/rot) but also means they remain in landfills for a long time.
Some additional polymers can be recycled by applying heat to remould the plastic into a new shape or break it back into monomers for repolymerization.
Why do addition polymers have high melting points
Addition polymers like poly(ethene) have higher melting points than their monomers because their long chains have many electrons, creating strong van der Waals forces.
Intermolecular forces in polymers 2
Additional polymers can be helped together by other intermolecular forces as well as van der waals. To figure out which one we can use the decision tree.
intermolecular forces in:
poly(ethene)
poly(chloroethene)
poly(ethenol)
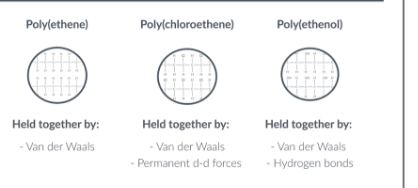
How do intermolecular forces affect the melting points of polymers?
When ranking the melting points of polymers remember that hydrogen bonding is the strongest bong, then dipole dipole and then van der waals.
So in the example poly(ethenol) has the highest melting point. Poly(ethene) has the lowest melting point
Why does addition polymerization occur and what conditions are needed?
Additional polymerization occurs because saturated hydrocarbons are more stable than unsaturated molecules. However, polymerization only happens under the right conditions, such as the presence of a catalyst.
Why does addition polymerization occur and what conditions are needed?
Additional polymerization occurs because saturated hydrocarbons are more stable than unsaturated molecules. However, polymerization only happens under the right conditions, such as the presence of a catalyst.
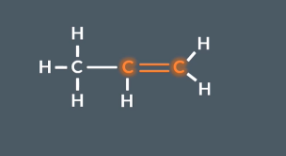
How do you convert a monomer into a polymer chain?
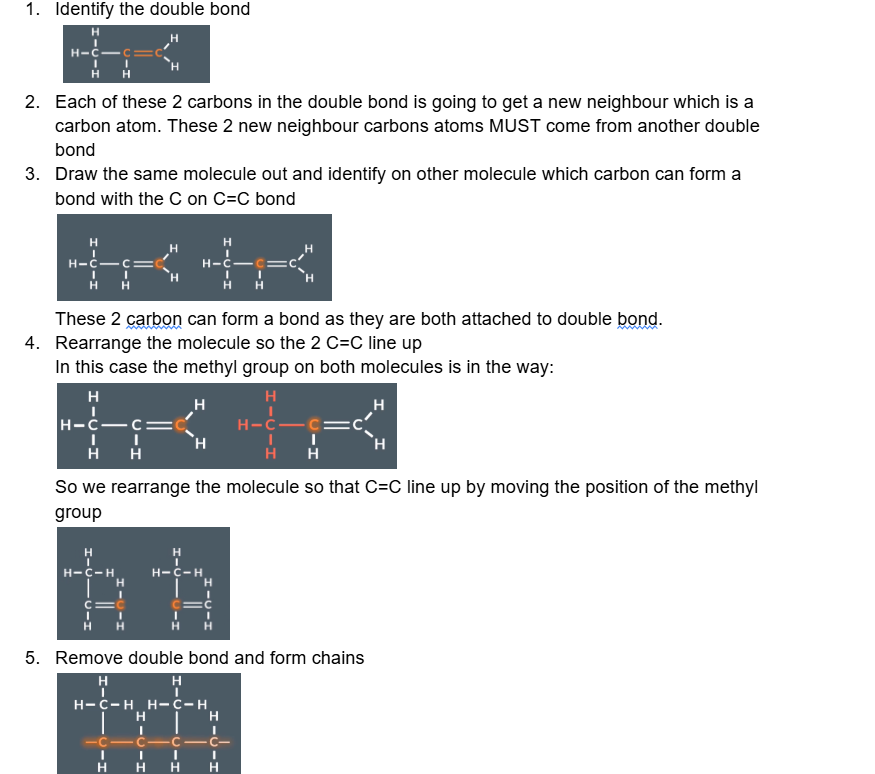
when drawing the polymer from the monomer
When turning a monomer into a polymer remember that the double bonded carbon (C=C) join together to form a new main chain. Anything else becomes a side chain
Identify the double carbon
Remember each of the carbon in the double bond will bonded to another carbon that used to come form a double bond
Rearrange the diagram if needed to line up the carbon atoms.
Replace the double bonds with single bonds.
Add dashes to each end of the molecule (where the carbons are) to indicate the repeating unit of the polymer chain
What is the repeat unit in a polymer, and how long can it be?
The repeat unit is the shortest section of the polymer that is repeated.
But by convention, we never show a repeat unit shorter than 2 carbons.
So even though in poly(ethene) the shortest repeating unit is H-C-H we show 2 carbons instead
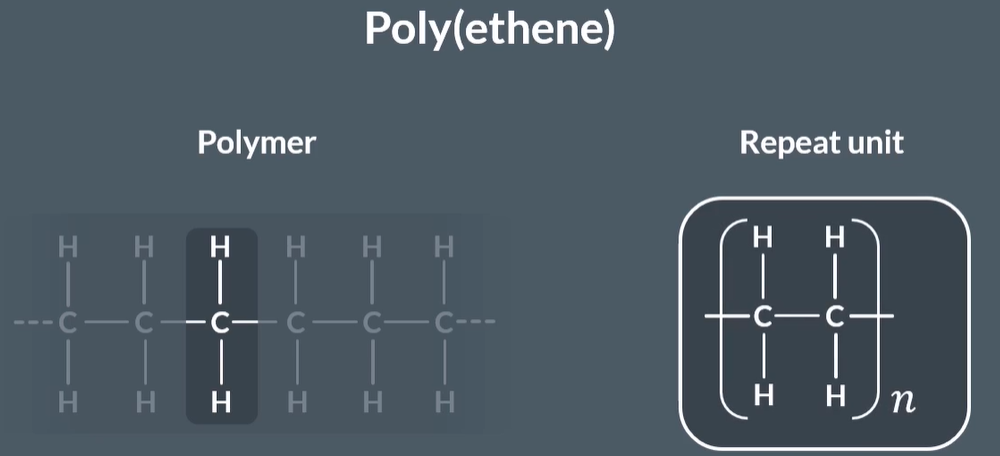
How do you identify and write the repeat unit of a polymer?
Split the polymer into pairs of carbon atoms.
Check that each pair has the same substituents.
Draw one of the pairs, showing bonds extending to indicate where the rest of the chain would be.
Add brackets around the repeat unit and add an "n" to indicate the number of repeating units.
Identifying the repeat unit
what is the repeat units for this polymer
Draw the shotest repeating unit (must be at least 2 carbons)
Remember to draw brackts and add n
Draw the repeat unit of poly(4-methylpent-1-ene).(practise)
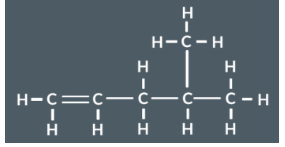
How to Split a polymer up into a monomer
Split the polymer up into pairs of cabron (2 carbons)
Check that each pair has the same substituents
Turn each pairs single bond into a double bond
Remove the bond between each pair and the next pair
Identify the monomer (E-but-2-ene)
pratice
pratcise
What should you do if the monomers are upside down or back to front in a polymer?
How to identity if a polymer is made of more than one monomer
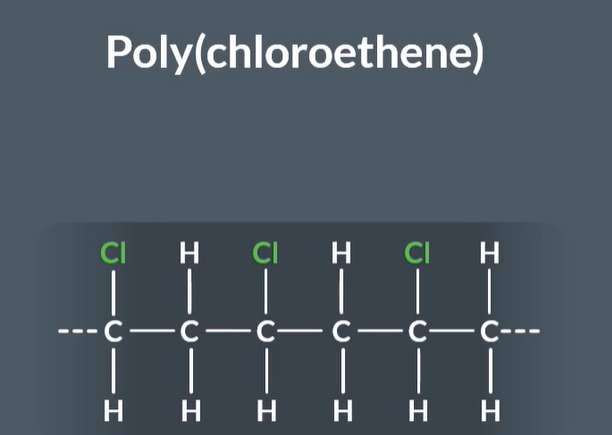
What is this polymer called and what are its properties
This polymer is called PVC or poly(chloroethene) and it has a very wide range of uses because it is rigid and flexible.
Uses include: drainage pipes,water supply pipes, sewage systems window frames, flooring (rigid) insulation on electrical wires and cables (flexible)
Why is PVC rigid
PVC is rigid because chlorine's electronegativity is higher than carbon's, so in a carbon-chlorine bond, the chlorine atom has a negative partial charge and the carbon atom has a positive partial charge. This makes the C-Cl bond polar, and the intermolecular forces between different chains of PVC include permanent dipole-dipole forces and van der Waals forces.
Plasticizers
When chemists don’t want PVC to be rigid, they add a plasticiser. The plasticiser acts by spacing the PVC chains apart, which reduces the intermolecular forces between them, allowing the material to bend rather than snap.
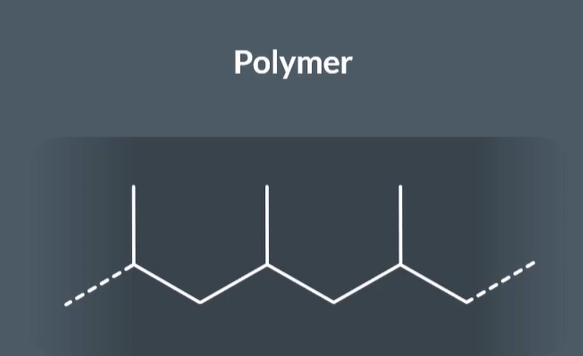
How do you handle polymer chains with different substituents when identifying the repeat unit?
In this example the pair does not have the same substituents. SO try splitting the polymer into 3 carbon and then 4 and then 5 to see if any have carbon have the same substituents. In this example if we split the polymer into 4 carbon the substituents all match
After this we can draw our repeat unit
YOU won't ever be asked to deduce the monomer of polymers like this however you can be asked to deduce the repeating unit of a polymer with double bonds
What should you do if a polymer is formed from a monomer with two double bonds and the exam asks for the repeat unit?
=
if a question asks for the repeat unit there is only 1 monomeer
The question will likely ask for the repeat unit from one monomer, so ensure you are correctly identifying the section that repeats.
What should you do if the exam asks you to draw the monomer for a polymer formed by two monomers?
If a polymer was formed by two monomers, then the exam is likely to ask you to draw the monomer. IF the question asks to draw the monomer - most likely tier will be 2 monomers THis is because we have a repeat unit for a polymer with no regular patten
Note
Repeat units unlikely to contain double bonds particulary C=C in main carbon chain
Monomers lkely to contain double bonds