Section 4 Endomembrane pt 2
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
What is a proteosome
Barrel-shaped protein degrading machines
What do proteosomes degrade?
Only proteins that have been tagged for destruction by ubiquitins
What parts make up a proteosome
Cap
Half a dozen proteases (to degrade proteins)
Several ATPases (to drive the process by hydrolyzing ATP)
What are the three enzymes required to add ubiquitin (small peptide)
E1 and E2 (ubiquitin carriers)
E3
What does the ubiqutin enzyme E3 do?
it is Ubiquitin ligase and recognizes misfolded proteins to transfer ubiquitin’s from E1 and E2 to the misfolded protein to mark it for destruction
Once a misfolded protein becomes polyubiquinated, where does it bind?
To the cap of the proteasome
What type of tag is for Lysosomes to know which to destroy?
M6P tag
What does BiP normally do?
Normally binds to misfolded proteins plus some BiP stays in the ER bound to sensors
What happens if there are many unfolded proteins
BiP will be recruited from the membrane and they will abandon their sensors causing the sensors to become active
When BiP leaves their sensors in the ER because there is too many unfolded proteins, what do some sensors do?
Some dimerize and add a phosphate to elF2alpha which will bind to the small subunit of ribosome and reduce protein synthesis
When BiP leaves their sensors in the ER because there is too many unfolded proteins, what do other sensors do?
Undergo Proteolytic cleavage and their cytosolic portion now acts as a transcription factor to make more proteins that alleviate stress
Which side of the golgi faces the ER
Cis golgi network
Who discovered the Golgi complex?
Camillo Golgi
What is the vesicular transport model of golgi transport?
The cisternae are stable compartments and cargo gets shipped from compartment to compartment and eventually leaves through the trans face
What is the cisternal maturation model of golgi transport (more widely accepted)
Cis cisternae are formed by fusion of vesicles from the ER and they mature as they move towards the trans face carrying cargo
What is proof #1 of the cisternal maturation model for the golgi
Cargo proteins were not found in vesicles
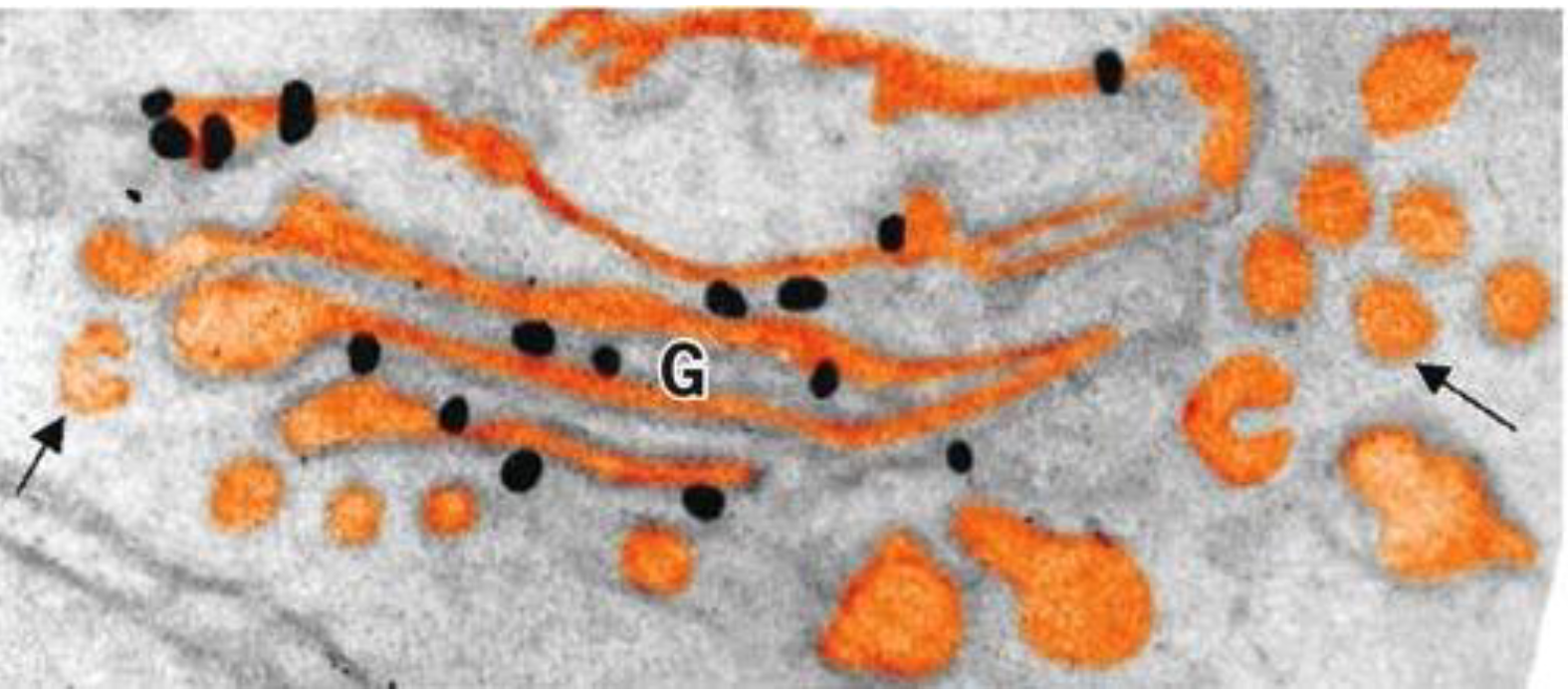
What is proof #2 of the cisternal maturation model for the golgi
Golgi resident proteins were seen in vesicles moving in the retrograde direction
What was proof #3 of the cisternal maturation model for the golgi
A yeast Sec mutant with a mutation in the ER vesicle formation had the golgi disappear when heated up
What are the three types of coats for vesicles?
COPII
COPI
Clathrin
What do coat proteins of vesicles act like?
a mechanical device that assemble to produce a force which will then curve the membrane until it forms a bubble
What is the origin, destination, and direction of COPII vesicles
originate from the ER and move towards the golgi; anterograde
What are the three types of coat proteins
Monomeric G protein (GTPase)
Adaptor proteins
Outer coat proteins
What is the first step of COPII vesicle formation
Sar1 (a GPTase) binds a GPT and becomes activated. Hydrophobic tail swings out of Sar1 and enters the lipid bilayer. This starts to curve the membrane
What is the monomeric G protein (GTPase) of COPII vesicles
Sar1
COPII vesicles: once Sar1 binds GTP and starts to bend the membrane, what happens?
It recruits two adaptor polypeptides (Sec23 and Sec24) to form a heterodimer which binds the cytoplasmic tails of transmembrane cargo receptors
COPII vesicles: after the adaptor polypeptides (Sec23 and Sec24) forma heterodrimes and bind the cytopalsmic tails fo the transmembrane cargo receptors, what happens?
Two COPII outer coat polypeptides (Sec13 and Sec 31) join complex to form an outer coat
What do the adaptor polypeptides of COPII bind to in total (Sec23 and Sec24)?
Tails of transmembrane cargo receptors
Sar1-GTP
Outer coat polypeptides
When do coat proteins of COPII vesicles disassemble?
When Sar1-GTP is hydrolyzed
What type of movement are COPI coated vesicles used for?
Retrograde movement:
Golgi to the ER
Trans golgi to the cis golgi
What monomeric G protein do COPI coated vesicles use
Arf1
What coat proteins do COPI coated vesicles use?
7 different proteins that form a coatamer which has a triskelion shape
How does the cell know which proteins to ship back to the ER?
KDEL which is a retrieval sequence
What is the vesicle formation of Clathrin Coated?
Triskelions with 3 heavy and 3 light chains that can join together to form a hexagon or pentagon
What can Clathrin Coated vesicles form?
Lattices
Which locations are Clathrin coated vesciles?
From trans golgi to endosomes/lysosomes—anterograde
From plasma membrane to endosomes/lysosomes—retrograde
How does clathrin coated vesicle formation often start?
Arf1 bending an alpha helix into membrane to initiate bending and recruitment of other adaptor proteins
What are the two adaptor proteins that can help recruit clathrin ?
GGAs from the trans golgi
AP2 from the plasma membrane
What is Dynamin and what does it do?
It is a monomeric G protein that uses GTP hydrolysis to help clathrin coated vesicles to leave the membrane
What happens when Dynamin is fed nonhydrolyzable GTPs
It will make a strand of dynamins without cutting off the vesicle
What is the optimal environment for the enzymes in lysosomes and what do they need to be tagged with to know to go to lysosomes?
acidic pH; Mannose 6 phosphate (M6P)
When a lysosomal enzyme is synthesized, what happens?
a carbohydrate is added as well as a phosphate which then binds to a M6P receptor and packaged in a clathrin coated vesicle until it comes into contact with the low pH of the late endosome
What are all the things GGA adaptor protein binds?
Mannose 6 phosphate receptor
Arf1 (GTPase)
Clathrin
What are the two ways you could break a monomeric G protein?
Nonhydrolyzable GTPs were used
Temperature sensitive sec mutants
What are Rabs?
Small GTP binding proteins that specify vesicle destination
In targeting vesicles to specific compartments, what does the targeting and tethering?
Rabs
In targeting vesicles to specific compartments, what does the docking?
SNAREs
What are SNAREs
membrane proteins that mediate vesicle fusion
What are the two type of SNARE proteins?
t-SNAREs = on the target membrane
v-SNAREs = on the transport vesicle membrane
How do Rabs associate with membranes?
Via a lipid anchor
How does Rab help vesicles combine into their specific compartments?
It stays attached to the vesicle via a lipid anchor and recruits tethering proteins to hold the vesicle in place so the two SNAREs can form a coil and pull the vesicle close to the target membrane
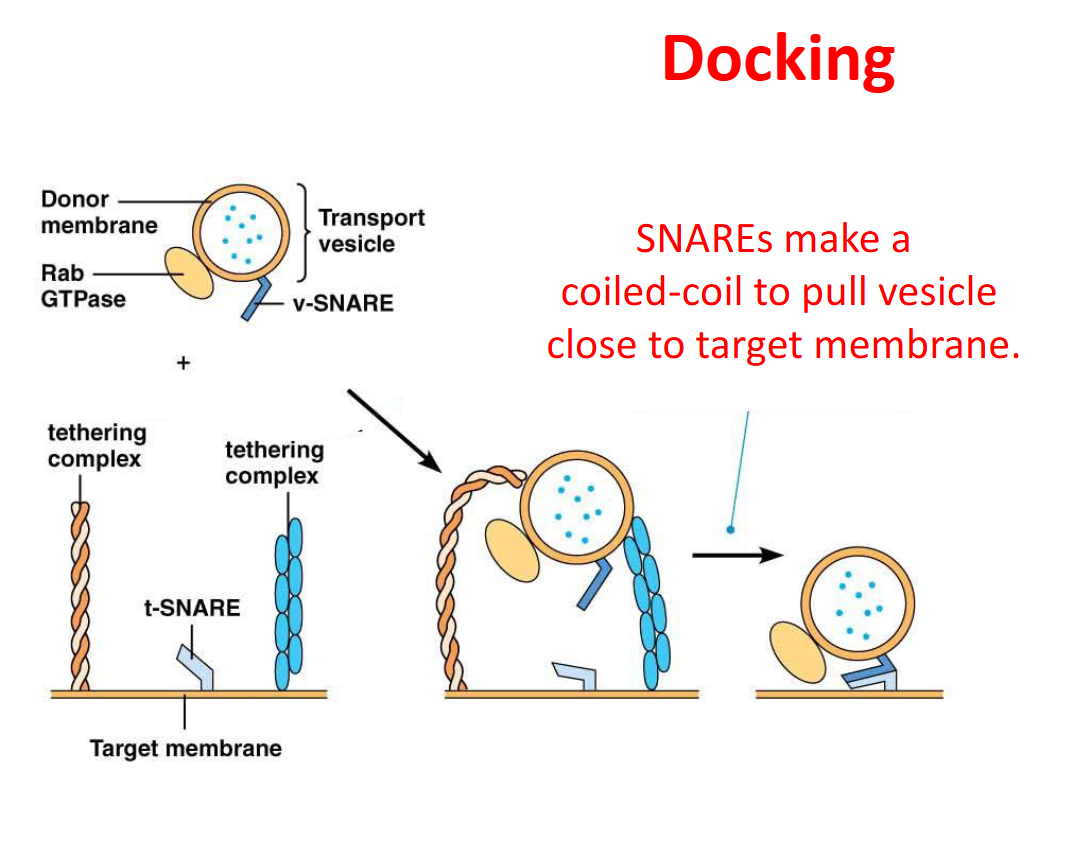
Once a vesicle fuses with the targeted membrane, what happens?
NSF uses ATP to untwist the SNAREs
What is receptor mediated endocytosis? (like yolk proteins taken up by a chicken oocyte)
Cell brings in extracellular materials after they bind to receptors on the plasma membrane (use clathrin coated vesicles)
How does the cell endocytose ligands?
Receptor mediated endocytosis. They bind to receptors on the surface of the plasma membrane and the more that bind, the more curved it gets until dynamin cuts it off and an endosome is created.
In receptor mediated endocytosis, after dynamin hydrolyzes GTP and causes the vesicle to pinch off of the plasma membrane, what happens?
Uncoating ATPase hydrolyzes ATP causing AP2 and Clathrin to be recycled
When a receptor mediated vesicle becomes an early endosome, whats the pH and what happens?
5.9-6.5; receptors are freed
When an early endosome becomes a late endosome, what is the pH and what happens?
5.5-6.0; can no longer fuse with endocytic vesicles
What are the two methods for acidification of receptor mediated endocytosis?
ATP-dependent proton pump
Transfer material to existing lysosome
What is receptor mediated endocytosis necessary for?
The uptake of housekeeping materials e.g., cholesterol uptake via LDL receptors
What is autophagy?
Destruction of organelles by isolation in a double-membraned vesicle followed by fusion with a lysosome
What did Yoshinori Ohsumi win the nobel prize for?
Discovering autophagy
What is the first step for how proteins get proteins into the mitochondria?
They have a transit sequence added Hsp70 and Hsp90 deliver proteins to the mitochondria in an unfolded state and they then go through TOM (transport outer membrane complex) in the outer membrane
Once proteins get through the TOM complex of the mitochondria, what does it do if it wants to lodge in teh inner membrane?
Goes into TIM22
Once proteins get through the TOM complex of the mitochondria, what does it do if it wants to get into the mitochondrial matrix?
It moves through the TIM23 complex with Mitochondrial Transit Peptidase cleaving the transit sequence while Hsp60 is added as a chaperone