CELL 201: Lecture 8 - Cell trafficking 1
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
92 Terms
Definition of Endomembrane system
Set of membranes that form a single functional and developmental unit
What are the components/what makes up the endomembrane system?
nuclear membrane
ER
Golgi
Lysosomes
Vesicles
Endosomes
Plasma membrane
Fill in the blank: The Endomembrane system is connected ____ or ____ through _____ transport
Connected DIRECTLY or INDIRECTLY through VESICLE transport
Where are proteins + lipids synthesized and incorporated into membranes?
ER
How are the proteins + lipids synthesized in the ER delivered to other compartments to form organelles?
Vesicular transport
What are the 2 types of transport related to vesicular transport?
Retrograde + Anterograde
Define Retrograde and Anterograde Transportation.
Retrograde = Vesicles from Plasma membrane back to ER
Anterograde = Vesicles moving towards the Plasma membrane
Why is there BOTH retrograde + anterograde Transportation/ BIDIRECTIONAL TRANSPORT? What is the importance of having both?
Bidirectional transport very important for balancing the flow of lipids and membrane proteins between organelles and plasma membrane to maintain the integrity of both.
as vesicles fuse with the plasma membrane + discharge their contents, the membrane (from the ER/Golgi) fuses with the PM + Becomes part of it
If all transport were anterograde, eventually there would be no more membrane for the ER or the Golgi organelles as all of it would be fused with the PM
The ER is a continuous network of what 3 things through the cytoplasm of a eukaryotic cell?
Flattened sacs
Tubules
Vesicles
What are the membrane bound sacs of the ER called and what is the space inside them called?
Membrane bound sacs = ER Cisternae
Space inside = ER lumen
What are the 2 basic kinds of ER that differ in structure and function?
Smooth ER
Rough ER
What is the main function of the smooth ER? Why is it smooth?
Lacks ribosomes = smooth = major site of MEMBRANE + LIPID synthesis
What is the main function of the Rough ER? Why is it Rough?
Has many sec61 translocon complexes (move proteins across or integrates it into ER) with Ribosomes = rough = major site of PROTEIN synthesis + insertion/transportation
Are the Ribosomes on rough ER on the luminal or Cytosolic side?
Cytosolic side of the membrane
What is the TRANSITIONAL ER (tER). Which ER membrane (smooth or rough) is it associated with? What ROLE does it play?
Located at the edge of the ROUGH ER
VESICLES FORMATION to shuttle lipids + proteins form
What type of structures do Rough + smooth ER form? aka. what shape are they
Rough = large FLATTENED sheets (Increase SA for ribosome docking)
Smooth = TUBULAR structures
True or False: BOTH types of ER are present in all cells in the same ratio amount
FALSE
both ER types are present in all cells
there is VARIATION in the relative amounts of each type of ER depending on the function of the cell
Cells with which types of functions are associated with each of the ER types?
Rough ER = Cells involved in the synthesis of Secretory proteins eg. Pancreatic cells
Smooth = Cells involved in the production of STEROID HORMONES eg. liver + endocrine cells
*****What is the general main function of the ROUGH ER?
Biosynthesis + Processing of proteins
*****What are the 5 Specific steps associated with the biosynthesis + processing of proteins done by the rough ER?
Co-translational protein import (sec62 translocon, signal peptidase etc.)
Folding of secreted + membrane proteins (chaperones, Protein Disulfide Isomerase etc.)
Addition of carbs to glycoproteins (glycosylation enzymes)
Recognition + removal of misfolded proteins (chaperones, ERAD etc.)
Assembly + budding of vesicles for transport to other compartments or for secretion
*****What is the general main function of the TRANSITIONAL ER?
Packaging of proteins into transport vesicles
assembly + budding of vesicles (sec12, sar1, COPII proteins)
In what shape/condition do proteins have to be to be moved into the tER?
Has to be properly folded before moved into the transitional ER
****What are the 5 functions of the SMOOTH ER?
Drug detox (liver)
Carb metabolism
Calcium storage
Steroid biosynthesis (lipid/hormones)
Membrane lipid synth
Drug detoxification often involves what CHEMICAL rxn/process and WHY?
Hydroxylation (adding OH groups)
Adding OH group to a drug increases solubility making them easier to excrete from the body
How can drug exposure affect the amount of Smooth ER?
it can upregulate the enzymes involve in this process and the smooth ER can proliferate
****What is the smooth ER involved in, in terms of carbs metabolism?
Break down of stored Glycogen
*****What is the enzyme unique to the SMOOTH ER that is involved in carb. metabolism?
Glucose 6 phosphatase
What does glucose 6 phosphatase do?
Hydrolyzes the phosphate from G6P to form free glucose that can be used in the blood
Is sER abundant in the liver? WHY?
Yes
Drug detox
Carb metabolism
True or false: Calcium pumps and channels are enriched in the smooth ER
True
for calcium storage
In what direction does the smooth ER pump calcium in terms of Calcium storage?
Actively pump from cytoplasm into ER for storage
Why does steroid synth occur at the smooth ER?
enzymes responsible for steroid biosynthesis are present at the smooth ER
True or false: The Golgi complex is functionally and physically linked to the ER
True
*****What occurs at the Golgi complex? What is its main role?
Glycoproteins + membrane lipids from the Er undergo Further processing + are sorted + packaged for transport
Main role: MEMBRANE + PROTEIN TRAFFICKING in eukaryotic cells
The Golgi consists of a series of flattened membrane bound _____
cisternae
A series of cisternae, usually ____ is called a Golgi stack
3-8
Some cells have one large and other have hundreds or thousands of Golgi stacks. Which cells have many stacks
Secretory cells
Each Golgi stack has ___ distinct sides or faces
2 faces
What are the 2 faces + differentiate between the 2 and what occurs at each face?
Cis = oriented towards the ER (cis-Golgi network CGN)
proteins + lipids are delivered to the CGN from the ER
Trans = On the other side of the ER (trans-Golgi network TGN)
Proteins + lipids destined for the cell surface or other organelles leave the Golgi
What do the Proteins + lipids leave the Trans Golgi complex in?
Transport vesicles that continuously bud from the TGN
Between the TGN + CGN are _____
Medial cisternae
What occurs at the Medial Cisternae?
much of the PROCESSING of proteins occur
Each compartment of the Golgi shows biochemical _____
Biochemical Polarity
What does it mean by biochemical polarity?
each compartment of the Golgi contains specific proteins unique to each portion of the network
some proteins are cis located and some are trans located
Much of the protein processing carried out in the ___ + Golgi involves _____?
ER + Golgi involves glycosylation
What is glycosylation
the addition of carbohydrate side chains to proteins
*****What are the 2 types of glycosylation
N-linked
O-linked
*****What occurs during N-glycosylation? what is added to what?
Addition of an oligosaccharide to the nitrogen atom of certain amino acid residues
******What specific amino acid residue does N-linked glycosylation connect the oligosaccharide to?
Asparagine residues
*****What occurs during O-glycosylation? what is added to what?
Addition of an oligosaccharide to the Oxygen of the OH group of certain amino acid residues
******What specific amino acid residue does O-linked glycosylation connect the oligosaccharide to?
Serine or Threonine
the ones with OH
Where does initial glycosylation occur?
In the ER
Which of the 2 types of Glycosylation N or O occurs in the ER?
N-glycosylation
All added carb chains initially have a common_______?
Core Oligosaccharide
What 3 things is the Common core oligosaccharide made up of?
2 units of N-acetylglucosamine
9 units of mannose
3 glucose units
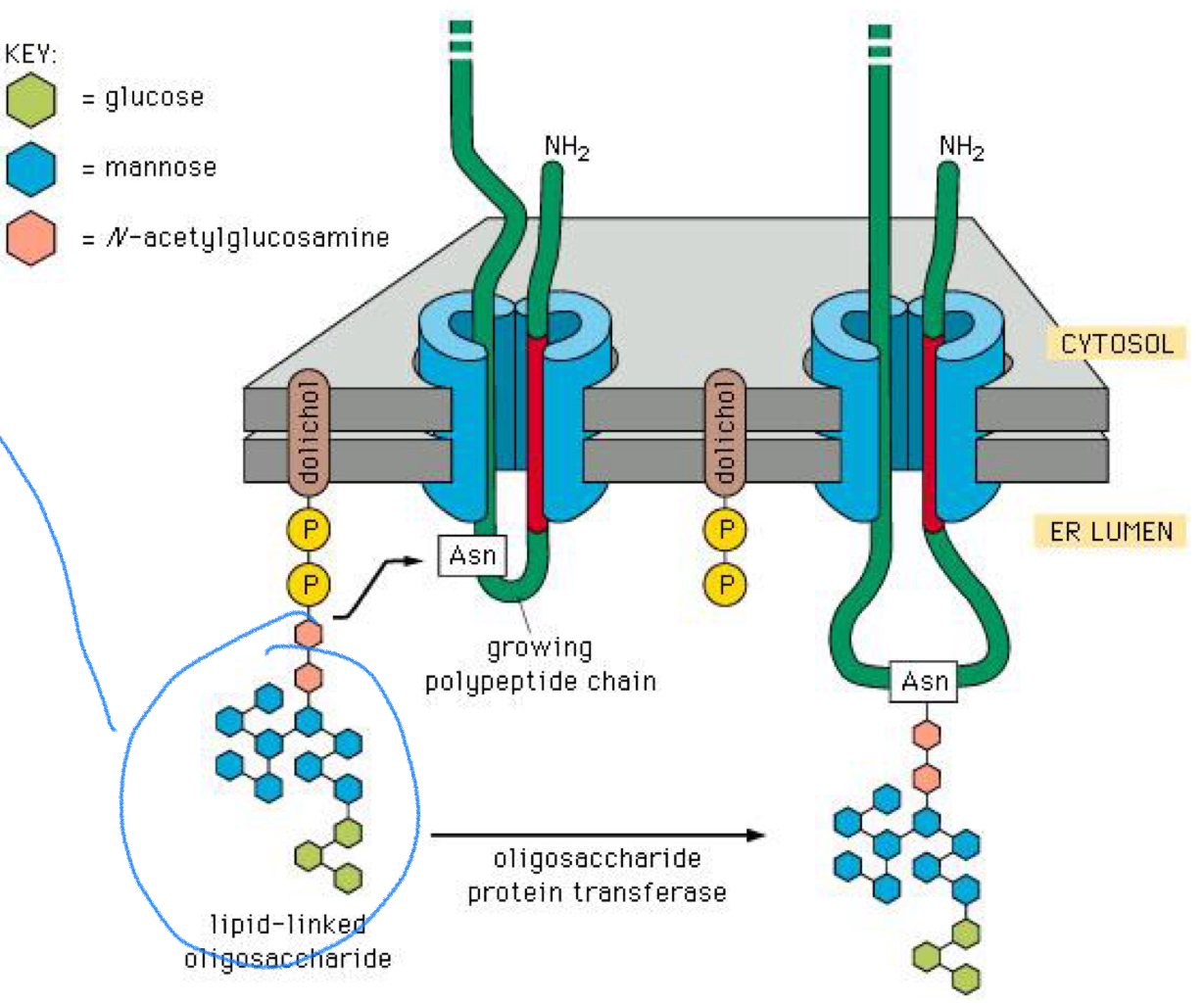
True or false: Glycosylation only occurs after the entire amino acid chain is fully translated
False
can occur co-translationally
What does co-translational glycosylation promote?
Proper protein folding
*****Explain the Role of GLYCOSYLATION in protein folding
2 ER enzymes act as chaperones for Monoglycosylated proteins + promote Disulfide bond formation (folding)
What are the 2 ER enzymes that act on MONOGLYCOSYLATED proteins?
CNX - Calnexin
CRT - Calreticulin
If the Core oligosaccharide of N-glycosylation initially consists of 3 units of glucose how does it become MONOGLYCOSYLATED?
Glucosidase 1 + 2 remove 2 glucose residues
Is the 3rd glucose of the core oligosaccharide ever removed?
yes after folding
When is the 3rd Glucose removed? What is it removed by?
Removed by Glucosidase 2 after release from CNX or CRT
What occurs of Protein folding is Incorrect?
One glucose unit is added back
What adds back the single glucose unit after incorrect folding?
Glucosyl transferase (UGGT)
What does the adding back of the single glucose unit do?
Make the A.A. chain a SUBSTRATE for CNX/CRT binding again to redo folding
What happens if It still isn’t folded correctly?
Cycle continues (adding back glucose, substrate for chaperone, release + removal of glucose) until the proper conformation is achieved + glucosyl transferase no longer binds
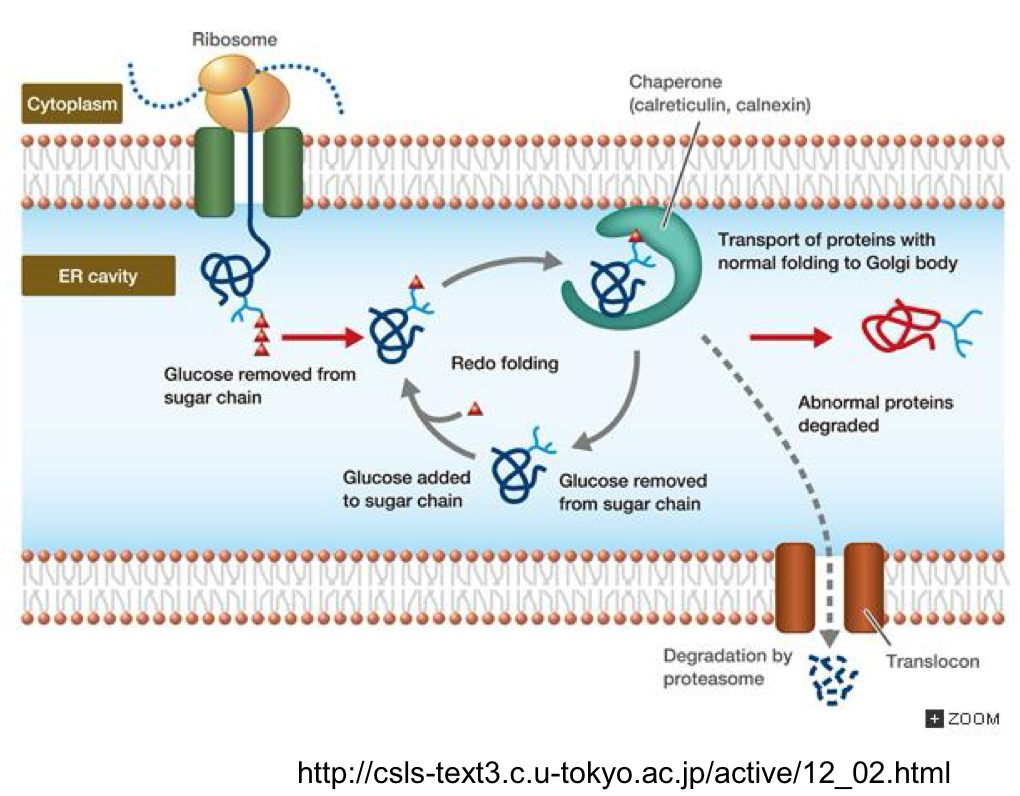
What is occurring at the same time as the glucosyl transferase activity that is competing with it?
ER Mannosidase 1 can bind + REMOVE a MANNOSE residue
What does the removal of the mannose residue by mannosidase 1 do?
Triggers ERAD
relocation/transport of aa out of ER to cytosol where proteosomes are present to degrade the misfolded protein
What ultimately determines the fate of the misfolded protein in the ER?
Competition between UGGT (glucosyl transferase) + Mannosidase 1
What occurs in the Golgi complex as N-glycosylated proteins from the ER move from the cis face to the trans face?
Further glycosylation + processing
How is great diversity in proteins created in the ER + Golgi?
N + O linked Glycosylation events are extremely VARIABLE
ER + Golgi contain hundreds of different glycosyl transferases
What are the 2 ways that proteins can be secreted out of the cell into the extracellular space?
Constitutive Secretion
Regulated secretion
Explain the process of Constitutive secretion in relation to the vesicles + give an EXAMPLE
Unregulated + Continuous + independent of external signals
After budding from TGN vesicles move Directly to cell surface + immediately fuse with PM
eg. Mucus secretion by intestinal lining
Explain the process of Regulated secretion in relation to the vesicles + give an EXAMPLE
Vesicles accumulate in the cells + only fuse with the PM in response to specific signals
eg. Neurotransmitter release
vesicles carrying neurotransmitters move close to site of secretion + remain there until receiving a signal (depolarization)
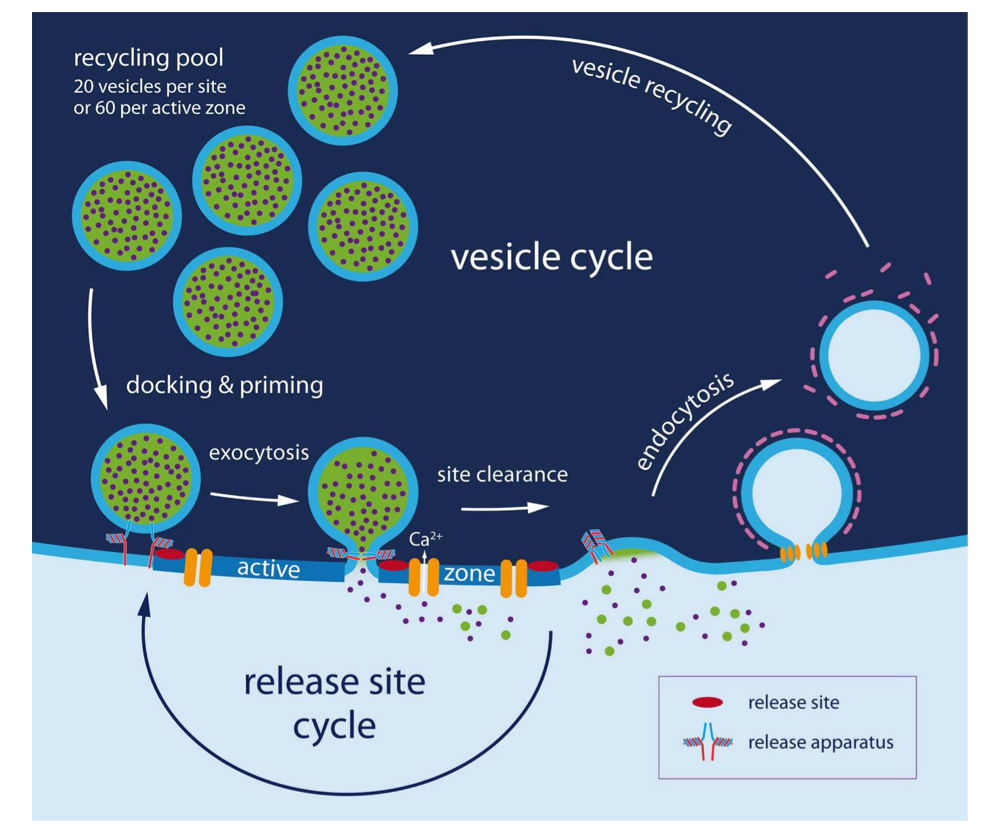
Why is Vesicle recycling/ vesicle cycle necessary?
replenishment of release-ready vesicles and sustaining neurotransmission during repetitive stimulation
because de novo synthesis of synaptic vesicles is too slow to replenish the pool
What does the mutant screen with budding yeast determine + how does it work?
Determines Genes + Proteins involved with secretion
Secretion mutants have a different density
Not able to secrete = build up = higher density
What is protein trafficking?
mechanisms that ensure proteins are delivered to their correct cellular destinations after they are synthesized.
Once a protein is at its destination it must be prevented from leaving
How is a protein trafficked to its correct location?
Proteins contain specific tag targeting it to a transport vesicle that will take it to the correct location
What are some examples of possible tags and what does it depend on?
A.A sequence, hydrophobic domain or oligosaccharide. side chain etc.
depends on the protein + the destination
What are the 2 possible functions of tags in relation the protein trafficking?
Take it to correct place + prevent it from leaving
Exclude material from certain vesicles
How is ER identity maintained?
By preventing some proteins from escaping the ER +/or by retrieving others from the Golgi
What are Retention/retrieval tags?
Tags that keep ER-specific transmembrane proteins in the ER or retrieve soluble proteins from the Golgi to the ER
What is the difference in the function of Retention/retrieval tags between Soluble + transmembrane ER proteins?
Transmembrane tag = Keep Protein localized to the ER
Soluble tags = Retrieve proteins if they travel to the Golgi + bring them back
*****What are the 3 tags that TRANSMEMBRANE ER localized/specific proteins contain?
RXR (arg-X-arg)
Dibasic
KK/ di-LYSINE
RR/ di- ARGININE
Where are KK tags located on the AA sequence and where are RR located?
KK = C-terminal
RR = Various locations
*****What are the 2 tags that SOLUBLE ER proteins contain?
KDEL (lys-asp-glu-leu)
HDEL (His-Asp-Glu-Leu)
What organism uses KDEL vs. HDEL?
KDEL = mammal
HDEL = Yeast
*****On which terminal of the AA sequence are soluble protein tags located?
C terminal
Under what conditions is a protein with a KDEL or HDEL sequence returned to the ER?
When it binds to a receptor in the ER its packaged into a transport vesicle for return to the ER
Evidence about the importance of retrieval tags comes experiments involving what?
Fusion proteins
joining of two or more genes that encode different proteins
If you want to see where protein goes what can you do?
Insert the GFP coding sequence
What does it mean by necessary vs. Sufficient
Necessary = required to have desired function
Sufficient = enough on its own to carry out the desired function