Carboxylic acids
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
A carboxylic acid is a molecule with…
an alcohol group bonded to a carbonyl group
What’s this molecule called?
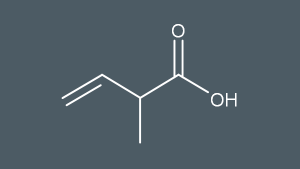
2-methylbut-3-enoic acid
When carboxylic acids react with carbonates, we observe…
effervescence caused by the production of CO2
Draw the carboxyl group
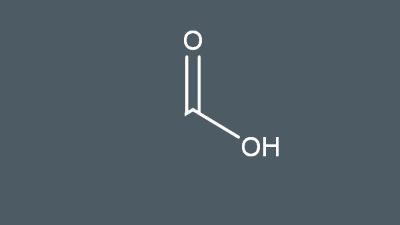
Carboxylic acid and water molecules can form…
hydrogen bonds
Two carboxylic acid molecules can form
hydrogen bonds and a dimer
melting point and solubility of a carboxylic acid like ethanoic acid?
quite a high melting point.
quite high solubility in water
Give a complete and balanced equation for the reaction between methanoic acid (HCOOH) and sodium.
HCOOH+Na—> HCOONa+1/2H2
Give a complete and balanced equation for the reaction between methanoic acid (HCOOH) and sodium oxide.
2HCOOH+Na2O—→ 2HCOONa+H2O
Give a complete and balanced equation for the reaction between methanoic acid (HCOOH) and sodium carbonate (Na2CO3).
2HCOOH+Na2CO3—> 2HCOONa+H2O+CO2
Carboxylic acids react with metals to form…
metal salts
hydrogen
Carboxylic acids react with metal oxides and hydroxides to form…
metal salts
water
Carboxylic acids react with metal carbonates to form…
metal salts
water
carbon dioxide
Draw the product from this reaction.
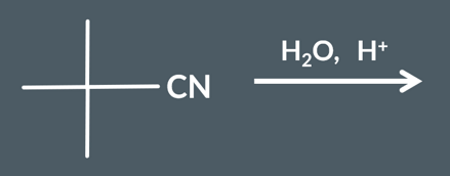
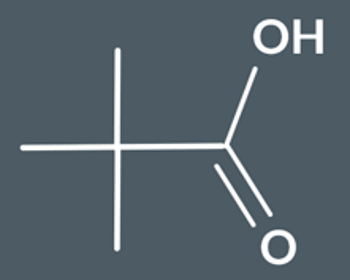
To convert a nitrile into a carboxylic acid, we need…
an acid.
water
What ion do we always produce when we convert a nitrile into a carboxylic acid?
NH4+
What type of reaction is the conversion of a nitrile to a carboxylic acid?
hydrolysis reaction
Propanoic acid and sodium hydroxide react to form…
sodium propanoate and water
Carboxylic acids ionise to form…
carboxylate ions.
Heptanoic acid and potassium hydroxide react to form…
potassium heptanoate and water
This salt is called...
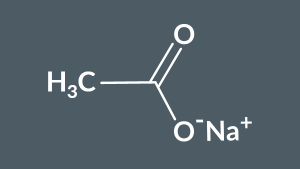
sodium ethanoate
Butanoic acid reacts with potassium carbonate to form…
Potassium butanoate, water and carbon dioxide.
Why do molecules act as acids when they have the carboxyl group, but not when they have separated alcohol and carbonyl groups?
This is because, in a carboxylate ion, the extra electron is shared over three atoms, making the ion more stable