Exam 3 patho
1/266
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
267 Terms
Main components of Blood Vessels
endothelial cells, smooth muscle, extracellular matrix- elastin, collagen, glycosaminoglycans
Categories of blood vessels based on size & structural function
Arteries, Arterioles, capillaries, venules, veins
Role of blood vessels
transport blood to tissues, regulate blood flow to tissues, control BP and secrete a variety of chemicals
Concentric layers of blood vessels
intima, media, adventitia
(most inner, middle, outer)
make up the tunica
play a role in blood pressure control
Arteries
convey blood from heart to capillaries moves away from the heart
-high blood pressure here
-thicker tunica media, narrower lumen than veins
-has more elastic and collagen fibers (spring back to shape)
-more resistant to change in blood pressure
Elastic Artery
Aorta- conduct blood from heart to muscle arteries -large proportion of elastic fibers allowing stretch and recoil
Muscular Artery
medium-sized blood vessels that distribute blood from the elastic arteries to the body's organs and tissues (specific body regions)
include most named arteries (brachial, coronary
vasodilation and constriction
Arteriole
a small branch of an artery leading into capillaries.
smallests of the arteries
sm is always somewhat constricted (vasomotor tone)
regulate systematic blood pressure and flow
Veins
transport blood from capillaries to heart
have thicker tunica adventitia and larger lumen than arteries
have less collagen and elastic fibers
walls collapse if no blood in vessels
Venules
smallest vein companion vessels with arterioles
smallest venules are postcapillary venules merge to form veins
small/medium veins
companion vessels with muscular arteries
Large veins
travel with elastic arteries
-numerous valves to prevent backflow (made of tunica intima)
Capillary
small vessels connecting arterioles to venules
small diameter optimal for exchange between blood and tissue fluid
Blood vessel disorders
Arterial disorders
venous disorders
Arterial disorders
hypertension
atherosclerosis
aneurysm and dissection
Vasculitis
Vasculitis blood vessel, hyperactivity
Venous disorders
varicose veins
normal blood pressure
120/80
systolic/diastolic
Hypertension
American college 130/90 mmHg
European 140/90 mmHg
Hypertension definition
persistently elevated arterial blood pressure greater or equal to 130/90 resulting from genetic and environmental factors
affects mostly small muscular arteries and arterioles
accelerates atherogenesis (increase plaque in the lumen of vessel) and causes degenerative changes in vessel walls (lg, med)
boarderline elevated htn
120-130 mmHg systolic
Types of HTN
Primary Htn (90-95% common)
Secondary HTN
Primary HTN
Essential or idiopathic hypertension
90 to 95% of individuals with hypertension
Caused by an increase in either both cardiac output, or total pressure resistance
CO=Heart rate(stroke volume-force)
This type of hypertension is multifactorial induced by genetic and environmental factors
primary htn mechanisms
renal mechanism, vascular, genetic, environmental
Renal mechanisms
insufficient NA+ excretion leads to increase in fluid volume leading to an increase in CO
“resetting of pressure natriuresis”
This term refers to the kidneys, inability to excrete sodium. The kidney decides it requires a higher pressure in order to excrete sodium, which maintains an increase in blood pressure
-in this scenario, the NA+ stays in the blood, which causes water to follow, causing h2o to stay in the system causing an increase in blood pressure and increase in CO
Vascular mechanisms
vasoconstriction + structural changes leads to increase in total pressure resistance (lumen size of vessel)
Genetic factors
single gene disorder leads to rare forms of hypertension
more than 500 genetic loci (susceptible genes)
Environmental factors
stress, obesity, smoking, physical, inactivity, heavy salt consumption
Treatment for hypertension
lifestyle changes
drug treatments
lifestyle changes
Exercise
Diet (decrease salt intake and increase weight loss)
eliminate stress
stop smoking
drug treatments
depend on many factors: dilate vessels and get rid of fluid
diuretics (pee out)
b1 blockers (decreases CO)
ACE inhibitors
calcium channel blockers
a1 blockers (prevents vasocontraction)
Secondary Hypertension
5-10% of individuals present
disease→ HTN
altered hemodynamic function due to primary disease condition
underlying conditions: renal, endocrine, cardiovascular, neurological diseases; example, renal artery stenosis, or other identifiable causes
rick eats chicken nuggets
Mechanisms of secondary hypertension
renovascular HTN
Primary hyperaldosteronism
single gene disorders
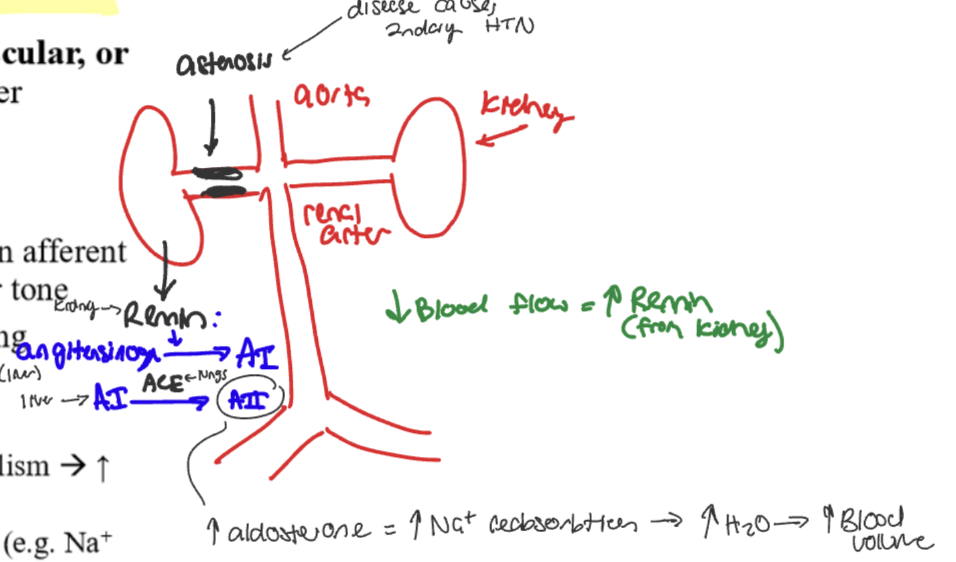
Renovascular HTN
RAS: decrease glomerular flow and pressure in afferent arteriole causes increase of renin secretion causes increase blood volume and vascular tone
Decrease blood flow froma disease like astenosis causes…
renin secreted @ kidney
activation of angiotensinogen @liver → Angiotensin I
Angiotensinogen I is activated by ACE @ lungs→ activation of angiotensinogen II
→ increase in aldosterone which causes an increase in Na+ reabsorption which causes increase water and increased blood volume
Primary hyperaldosteronism (part of secondary htn)
idiopathic or aldosterone secreting adenomas
increase in aldosterone which causes NA+ reabsorption-aldosterone comes from the adrenal gland .
Single gene disorders
gene defects affecting enzymes involved in aldosterone metabolism causing an increase in aldosterone which leads to an increase in aldosterone in the vessels
-mutations affecting proteins that influence sodium reabsorption, for example if the body can’t regulate NA+ channels causing an increase in reabsorption
Underlying diseases that lead to secondary HTN
renal, endocrine, cardiovascular, neurologic
Atherosclerosis
plaque build up
-progressive disease that affects the arteries
mainly elastic and muscular arteries
characterized by accumulation of plaque leading to narrowing vessel lumen
What is the problem with plaque?
atherosclerosis is characterized by a buildup of plaque so this causes narrowing lumen of blood vessels.
risk factors can lead to intimal lesions called atheromas or atherosclerotic plaques that protrude into the vessel lumen
what happens?
-obstructing flow
rupture→ thrombosis
thickened wall→ischemic injury and weakened media wall leading to aneurysm formation
Risk factors for atherosclerosis
Acquired factors, inherited factors, gender- and age associated factors
Acquired factors
hypertension, cigarette smoking, hyperlipidemia (low density, lipoprotein, or LDL) diabetes, inflammation
Inherited factors
Family history, and genetic abnormalities
Gender and age factors
increasing age and male gender
Atherosclerotic plaques consists of
fibrous cap and necrotic center
tunica intima is broken
fibrous cap
smooth muscle cell, macrophages, foam cells, lymphocytes, collagen, elastin, proteoglycan, and neovascularization
Necrotic center
cell debris, cholesterol, crystals, foam cells, calcium
foam cells
macrophage that have endocytosed lipid cells
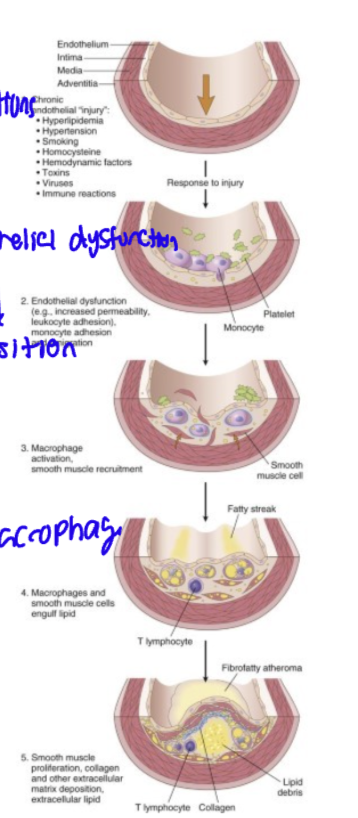
Pathophysiology of atherosclerosis
endothelial injury→ chronic inflammatory and healing response
lesion progression involves LDLs, macrophages and T cells interacting with endothelial and smooth muscle cells of the arterial wall
Simple terms patho of Atherosclerosis
chronic condition (endothelial injury) → endothelial dysfunction→macrophage activation→SMC/ Macrophage engulf lipid→deposition →plaque thickens
Complications of atherosclerosis
MI -heart attack
cerebral infarction (stroke)
aortic aneurysm (wall weakens and bulges)
peripheral vascular disease- narrowing periphery and lymph vessels
Mesenteric occlusion
chronic ischemic heart disease
sudden cardiac death
ischemic encephalopathy (brain tissue)
Vasculitis
information of vessel, walls, it affects vessels of certain caliber and location,
frequently associated with fever, myalgias, arthralgias, organ dysfunction
Common mechanisms of vasculitis
immune mediated inflammation-complex deposition
Direct invasion of vascular walls by infectious pathogens
Vasculitis diseases
Granulomatous disease (giant cell arteritis)
Kawasaki disease
Churg-Strauss syndrome
Buerger disease
Giant cell arteritis - Granulomatous disease
large vessel vasculitis
common in r temporal region
must be treated with steroids right away
facial pain/ headache, ocular symptoms
Aorta +
affects temporal, vertebral and ophthalmic arteries
greater than 40 yrs with or without polymyalgia rheumatica
Kawasaki disease
medium vessel vasculitis in the arteries
anti-endothelial cell antibodies in a young child
symptoms
Fever for five days, cervical lymph nodes, greater than 1.5 cm, rash, bilateral conjunctivitis, coronary artery aneurysms(long term problem), swelling of palms and soles of feet, mucositis of the mouth
Churg-Strauss syndrome
small vessel vasculitis
presence of asthma and allergies, increased levels of eosinophils in blood vessels.
eosinophilia, asthma, and granulomas
skin and kidney problems, immune complex mediated, presence of purpora
small arteries
eosiniphils req.
asthma and atopy (genetic predisposition to developing allergic conditions, such as asthma, eczema)
Buerger disease
thromboangiitis obliterans
thrombosis req
young male smoker
Specifics of thromboangiitis obliterans
Buerger’s Disease
vasculitis of the small and medium sized arteries in the upper and lower extremities
-this disease is characterized by lack of perfusion/oxygenation of the tissue
tibial and radial arteries
inflammation leads to thrombosis, fibrosis and scar tissue formation
-can affect the nerves as well, strongly associated with cigarette smoking (95% of cases)
Symptoms: pain at rest, claudicatio (only pain when they are doing something) , venus inflammation
Complications: ulceration, gangrene, amputation
Treatment: stop smoking at earlier ages
Blood vessel hyperactivity
vasospasm and contraction
Raynaud Phenomenon
bv hyperactivity
exaggerated vasoconstriction of arteries and arterioles in response to too cold or emotional
Extremities: fingers, toes, earlobes, nose or lips
Mechanism vasoconstriction→ restricted blood flow→ pallor (pale white)and cyanosis (purple /blue, lack oxygen
Primary raynaud phenomenon
young womenonly disease affecting pt
symmetrically affecting the extremities (both limbs)
triggered by intrinsic hyperactivity of the smooth muscle cells which contract and cause narrowing of the BV
secondary raynaud phenomenon
caused by another disease such as SLE, scleroderma, buerger disease, or atherosclerosis
asymmetric involvement of extremities that progressively worsens over time
Varicose veins
abnormally dialated veins, prolonged increased intraluminal pressure and by loss of support of the vessel wall \
occurs in superficial veins of the upper and lower leg
ex. the great saphenous vein can become so dilated that it can no longer do its job
causes, s/s, tx. of varicose veins
increase in localized venus pressure- prolonged standing or pregnancy/obesity
s/s- aching, swelling→ stasis dermatitis, ulceration→ poor wound healing
tx. stockings or surgery
what is stasis dermatitis: pooling of blood in the veins
varicose veins at the esophageal varices
varicose vein that develops in the lining of the esophagus. Their formation is primarily driven by portal hypertension, an increase in blood pressure within the portal venous system
This elevated pressure causes blood to bypass the liver through collateral veins, leading to their dilation and tortuosity, particularly in the lower esophagus
The veins connecting the portal system to the systemic circulation in the lower esophagus are particularly susceptible to this increased pressure. As blood is shunted through these veins, they become engorged, dilated, and tortuous, forming esophageal varices. The increased intraluminal pressure causes these veins to bulge into the esophageal lumen
often if the liver can’t function , blood moves backwards in systemic circulation causing varices that can cause the esophagus to easily tear
hemorrhoids
occur in the anal and rectal area. Their development is fundamentally linked to the same mechanisms that cause varicose veins elsewhere in the body: prolonged, increased intraluminal pressure within the veins and a loss of support in the vessel walls. This leads to the abnormal dilation and tortuosity characteristic of varicose veins
Increased Localized Venous Pressure: Elevated pressure within the veins of the anal canal and rectum is a key factor. This pressure can be exacerbated by activities that strain the abdominal and pelvic regions
Prolonged Standing: Similar to varicose veins in the legs, prolonged standing can increase pressure in the venous system, including the pelvic veins, which contributes to the development of hemorrhoids
Pregnancy/Obesity: These conditions significantly increase intra-abdominal pressure, which in turn elevates venous pressure in the lower body and pelvis, predisposing individuals to both lower extremity varicose veins and hemorrhoids
Cardiovascular system
heart, blood vessels, blood
Circuits of the cardiovascular system
heart pumps blood via the pulmonary and systematic circuits
Pulmonary circuit
blood moves from the pulmonary artery at the right side of the heart to the lungs, where the blood becomes oxygenated, then the blood moves through the pulmonary veins into the left side of the heart (atrum to ventricle)
Systematic circut
blood moves from the left ventricle into the aorta then blood is perfused around the body and blood re-enters the heart via the vena cava and moves into the right atrium
Six mechanisms that lead to a broken heart
failure of the pump
obstruction to flow
regurgitant flow
shunted flow
disorder of cardiac conduction
rupture of the heart or major vessels
Congestive heart failure
inability to effectively pump blood to meet the metabolic demands of the tissues
or req. elevated filling pressures
Etiologies that lead to CHF
ischemic heart dz (coronary vessels are blocked)
Hypertension
valvular heart disease
arrhythmias
congenital defects
cardiomyopathy (thickening of the heart muscle, deposition of proteins that prevent normal function
Cardiac hypertrophy
increase in size of cells of the heart
causes progression to CHF
Patho of cardiac hypertrophy
sustained increase in mechanical work (pressure/volume overload) leads to cell hypertrophy
the heart tries to maintain CO so therefor it has to work harder
increase in DNA ploidy- increase in chromosome sets in the heart cells
Pattern of hypertrophy
increase in wall thickness and mass→ sarcomeres parallell→ pressure overload
increase in mass→sarcomeres assembled in series→ volume overload
Patho of cardiac hypertrophy
increase in mechanical work
changes to gene expression, HR/contractility, oxygen supply, fibrous tissue deposition
Hemodynamic changes and circulatory problems
Brain natriuretic peptide amd ECG used to assess extent of CHF
Hemodynamic changes and circulatory problems in CHF
decrease in CO, BP, tissue perfusion (forward failure (to body))
pooling of blood (backward failure (back to heart toward lungs))
Left sided Congestive heart failure
most often caused by IHD or HTN, aortic and mitral valvular disease or primary myocardial disease
passive congestion (blood backing up, stasis of blood in the chamber and inadequate tissue perfusion
compensation: catecholamine release (NE/E), RAAS, ADH (antidiuretic hormone)
Left sided CHF Key
Backward effect: backing uo of blood to lungs (cause shortness of breath)
increase pressure behind pump leads to pulmonary congestion
Forward effect: low cardiac output to body (dilation of left ventricle and aorta)
organs involved: heart lungs kidney brain
Mechanism/ compensation with L CHF
Pt. 1
Myocardial dysfunction: there is a presence of a condition that prevents blood from being pumped out of the heart
-MI, IHD, HTN
As a result there is a decrease in CO and systemic BP bc blood is not being pumped out of the heart.
Baroreceptors activate and detect pressure change (located at left ventricle, aortic arch, or carotid sinus)
These receptors then signal the medulla in the brain’s to activate the sympathetic system and trigger the adrenal gland to produce catecholamines (NE/E)
These hormones cause vasoconstriction and lead to an increase in afterload, BP, and heart rate
this causes ventricular remodeling (Hypertrophy and dilation of the ventricle-genetically large cells with impaired contractility)
PT 2.
Myocardial dysfunction: there is a presence of a condition that prevents blood from being pumped out of the heart to the kidneys
-MI, IHD, HTN
When blood is not pumped to the kidneys, the kidneys produce renin, which triggers the RAAS pathway
Renin activates angiotensinogen - Angiotensin I @ liver
ACE @lungs activates Angiotensin I- Angiotensin IIAngiotensin II goes to the adrenal cortex and aldosterone is produced.
Aldosterone causes an increase in NA+ reabsorbtion which leads to in increase in H2o and blood volume
Result: increase in Blood volume (afterload), vasodilation, HTN, heart rate
leads to ventricular remodeling
Left sided dysfunctions
systolic
diastolic
Systolic dysfunction
inability to contract/ pump
reduced contractibility of LV→ decrease in CO and BP→ inadequate tissue perfusion
Diastolic dysfunction
inability to fill (relax)
LV abnormally stiff and can not relax during diastole
any increase in filling pressure→ pulmonary congestion
exacerbated with increase in metabolic demands
Etiology- HTN> DM, obesity, renal artery stenosis
Right sided heart failure
inability of the right side of the heart to pump blood to the lungs.
most common cause is left sided CHF, causing pulmonary congestion.
Isolated right-sided CHF can be due to a variety of lung disorders, like primary pulmonary HTN
Core pulmonale- diagnosis when RCHF is due to another condition like COPD, pleural effusion, or cancer
Right CHF flow
Backward: to systemic congestion of the body (pooling of blood at body)
Forward Low cardiac output to lungs
Organs: heart liver portal system, spleen, kidney, subcutaneous tissue, brain
often JVD is seen
Ischemic heart disease
a group of syndrome caused by lack of oxygenation, nutrients, and removal of metabolic waste
Greater than 90% are due to coronary atherosclerosis
less common causes: coronary emboli, myocardial vessel inflammation, vascular spasm (smooth muscle contraction)
Progression: begins silent→ sudden onset of symptoms
Some people may not realize they have this condition because the obstruction takes time to build up
CAD
coronary artery disease- coronary athersclerosis (vessels of the coronaries)
involves LAD, RCA, LCX
left descending, left circumflex, right coronary artery
Clinical manifestation of IHD
Angina pectoris- chest pain
myocardial infarction (heart attack)
chronic IHD with heart failure (lack of pumping)
sudden cardiac death (SCD)
Angina Pectoris
characterized by paroxysmal and recurrent attacks (occur quickly and uncontrollably) location: substernal or precordial (infront of heart) chest discomfort
Transient ischemia
not severe enough to cause necrosis (15 seconds to 15 minutes)
Three patterns of angina
stable (typical) angina
Prinzmetal variant angina
unstable (crescendo) angina
Stable (typical angina)
some obstruction from narrowing of coronary artery causing insufficient blood flow to the heart
stable angina often leads to unstable angina
most common caused an imbalance and coronary confusion related to demand: how much blood the heart gets vs how much it needs
Associated with underlying CAD
Pain on exertion or increased demand, for example, pain could increase with extreme emotions or exposure to the cold
Type of pain:
Crushing or squeezing pain that radiates
Relieved by rest or vasodilators
Often described as "left sided pain “
Prinzmetal variant angina
Has nothing to do with plaque, part of vasospasm
This is a type of episodic ischemia caused by coronary artery spasm, this comes and goes, and has nothing to do with atherosclerosis
Unstable crescendo angina
progression from stable angina it has more long-term obstruction, often longer than 20 minutes of prolonged pain and frequency
Precipitated by less exertion or rest
Associated with plaque disruption and superimposed thrombosis (tear of vessel causes thrombosis), embolization of thrombus and or vasa spasm
Embolization of a thrombus is when the thrombus moves through circulation and gets caught causing pain
high risk of MI
NEXT STEP- MI
Myocardial Infarction
death of cardiac muscle due to prolonged ischemia
Referred to as heart attack
10% occurs in less than four-year-olds, 45% occurs in less than 65-year-old
Majority occur in the left ventricle
Risk factors MI
increasing age
Male gender
Post menopausal women-estrogen is a protective feature
Increase atherosclerotic risk factors (genetics, age, smoking, male, hypertension)
Mechanism MI
90% relates to this mechanism
Atheromatous plaque disrupted lead to hemostasis (clottong) activated, causing completely occluded vessels
Other mechanisms of MI
Vaso spasm- with/without atherosclerosis, platelet, aggregation, or drug ingestion like cocaine
Emboli-thrombus (blood clot) circulating in the blood that comes from somewhere else
Uncommon causes include vessel disorders, like vasculitis, hematologic abnormalities, amyloid deposition in walls, and vascular dissection
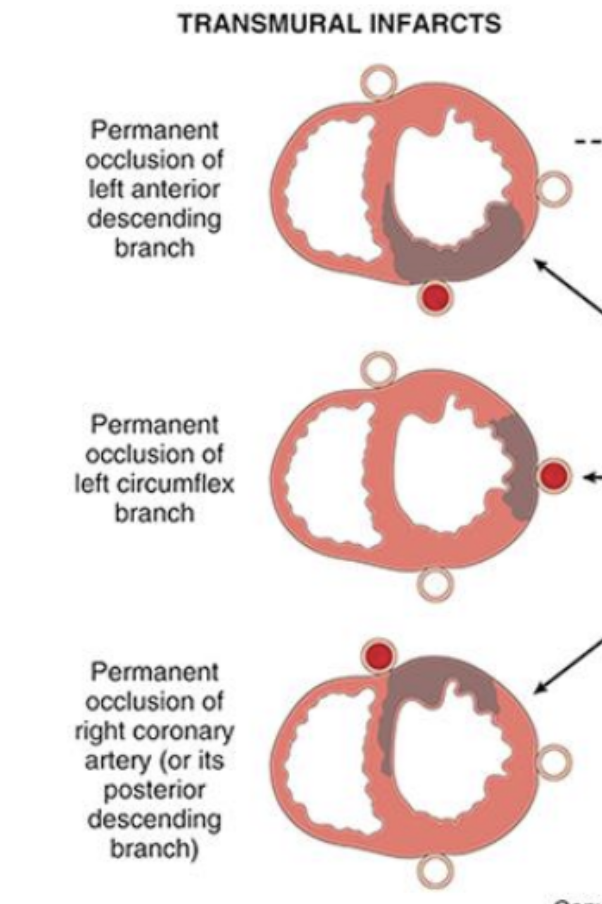
Patterns of infarction
myocardial, necrosis correlates with the location of the infarction
Types include transmural, infarction, and sub, endocardial infarction
Transmural infarction
The whole vessel is obstructed
Occlusion of epicardial vessels
Necrosis involves full or nearly full thickness of ventricular wall
(across whole vessel wall)