Chem Unit 2 & 3 test
1/34
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Atomic Number
number of protons in the nucleus of an atom, identifies the element
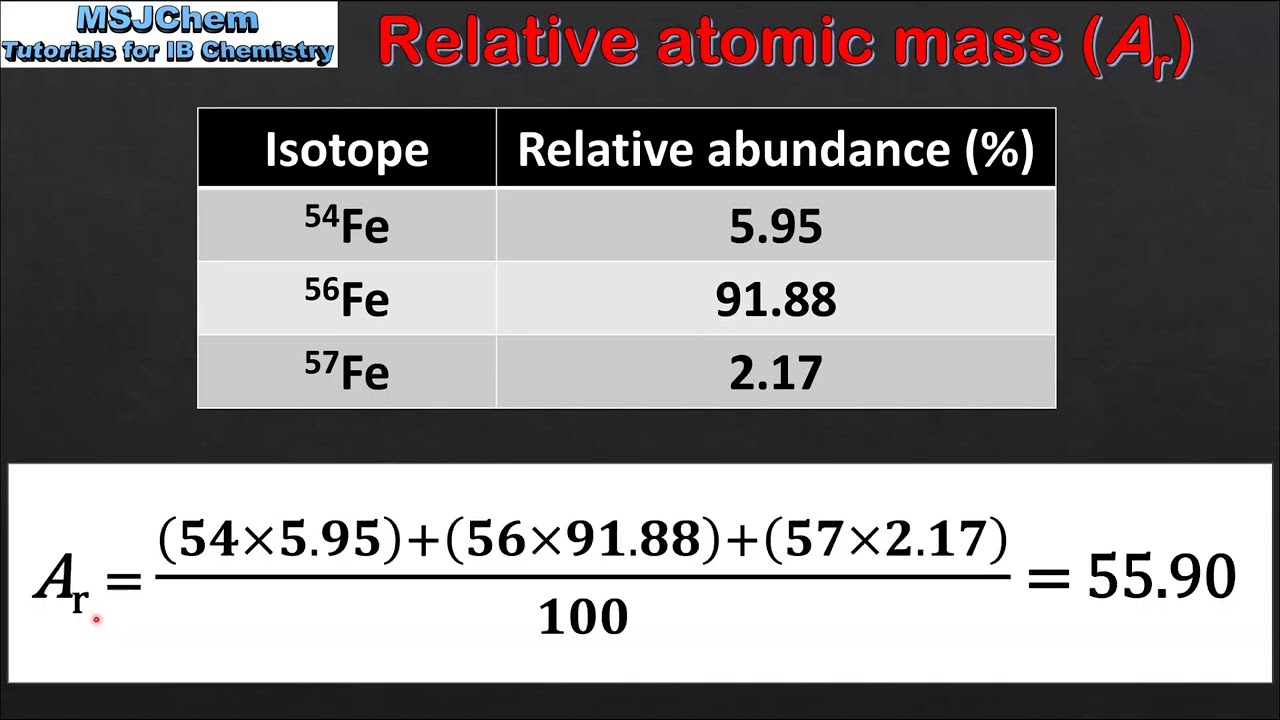
Calculating Atomic Mass
atomic masses are weighted averages that take into account the natural abundances of each isotope
Mass number
the protons and neutrons in the nucleus. Equal to the number of nucleons. Identifies an isotope, always a whole number
Isotope
atoms of the same elements with a different number of neutrons. Mass number and number of nucleons will be different as well. Same atomic #, different mass # or same # of protons, different # of neutrons
Isotopic notation
symbol of the element,the element’s atomic number and the mass number
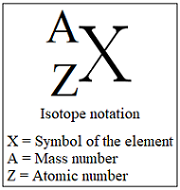
Ion
a charged particle formed by the loss or gain of an atom’s valance electrons
Dalton Model (1803)
The atom is a solid indivisible sphere, also known as “Billiard Ball” model

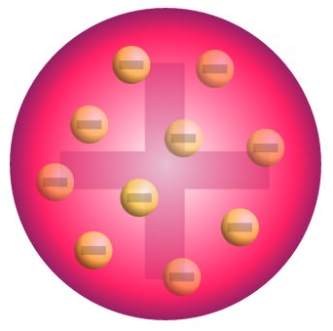
Thompson Model (1897)
known as the Plum Pudding model, discovered the electron. The atom is a sphere with small electrons embedded in a positively charged mass
Experiment: Cathode Ray Tube
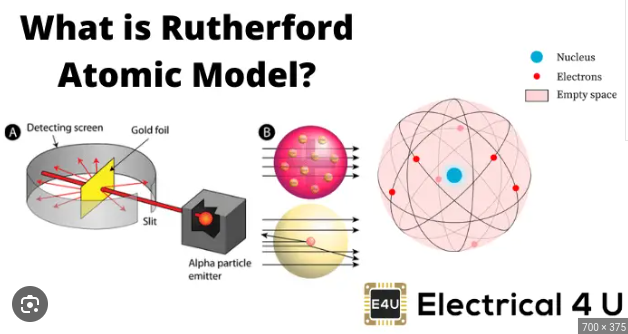
Rutherford Model (1911)
The atom is mostly empty space with a small dense nucleus with electrons moving around it like the planets move around the sun.
Experiment: Gold Foil
Bohr Model (1913)
The electrons in an atom move in circular fixed orbits at fixed distances from the nucleus. Problem is that this model only worked for explaining Hydrogen
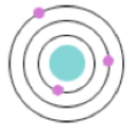
Electron Cloud/ Wave Mechanical Model/ Modern Model (1926)
Introduces quantum mechanics to the world, describes electrons as both waves & particles

Orbitals
region of high probability of finding an electron
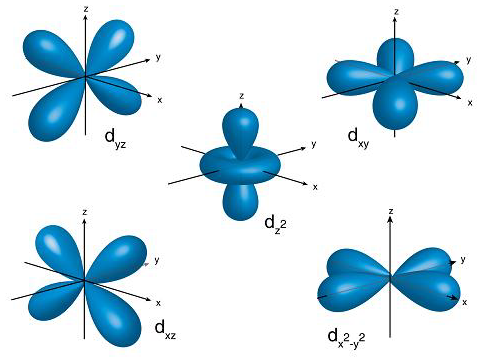
Electron Configuration Rules
Aufbau Principal: electrons occupy the orbitals of lowest energy first
Pauli Exclusion Principal: no two electrons can occupy the same space in an orbital
Hund’s Rule: each orbital can hold 2 electrons - fill each orbital /w 1 electron before pairing electrons in an orbital
Electron Configuration
electrons are distributed in the electron cloud into principal energy levels (1, 2, 3..), sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (2 electrons allowed per orbital)
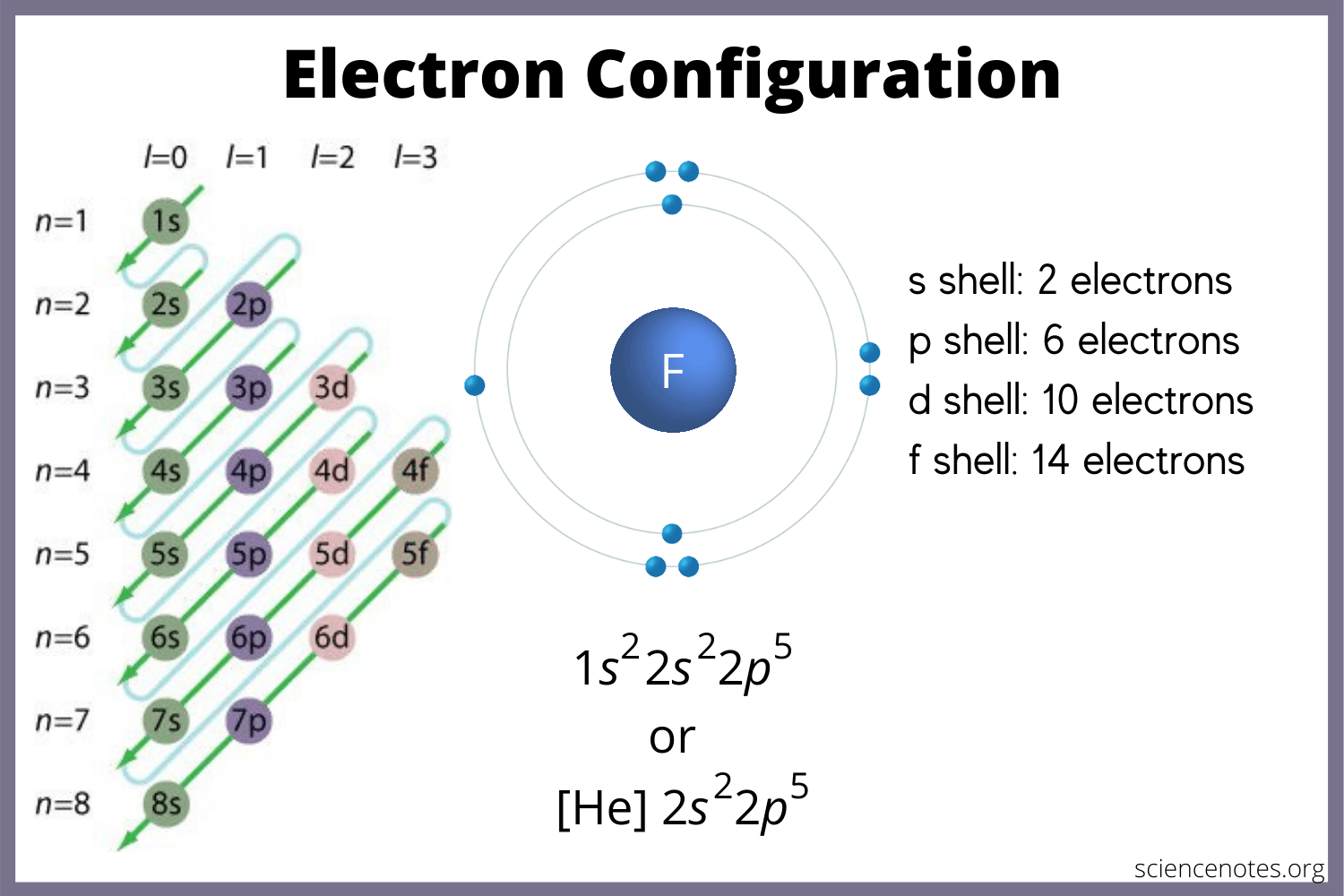
Valance Electrons
-the electrons in the outermost energy level
-the only electrons in a chemical reaction, # of electrons determine how atom will react
-Lewis/Electron dot diagrams show valance electrons only

Cation
an atom with 1, 2 , or 3 valence electrons will lose those electrons to become a stable ion, a positive ion is called a cation
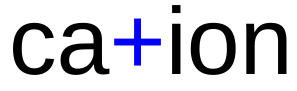
Anion
an atom with 5, 6, or 7 valence electrons will gain electrons to become a stable ion, a negative ion is called an anion

Group
Vertical columns on the PT where all the elements have the same # of valance electrons, have similar properties and the size of atoms increase as you go down a group
Period
Horizontal rows on the PT where elements have the same # of energy levels
size of atoms decrease as you move across period
Electronegativity
the ability to attract electrons in a chemical bond
Ionization Energy
energy required to remove a valance electron from an atom
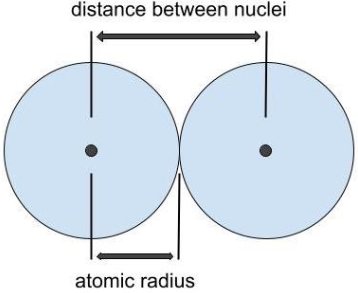
Atomic Radius
Size of an atom, measured by the distance between two nuclei of the same atom
Atomic radius increases as you go down a group because there are an increasing number of principal energy levels
Nuclear Charge
Metal
elements to the left of the staircase, except hydrogen. Properties of metals include luster, conductivity, malleability, ductility, and losing electrons easily
Non-Metals
elements to the right of the staircase, plus hydrogen. Non-metals are dull, brittle, nonconductors ans gain electrons easily
Metalloid
6 elements that border the staircase, they have metallic and nonmetallic properties simultaneously. Often uses in technology because they are semiconductors
Transition Metal
these elements in groups 3-12 are brightly colored solutions and have multiple positive oxidation numbers, less reactive than groups 1 and 2
Alkali Metal
most reactive metals on the PT, contain one valance electron and can not be found in nature b/c of their reactivity
Alkaline Earth Metal
metals in group 2 that contain 2 valance electrons. Can not be found in nature but are less reactive than alkali metals
Halogen
nonmetal elements in group 17 and each element contain 7 valance electrons. This is the only group containing elements that exist in all 3 phases (F2 and Cl2 are gases, Br2 is a liquid, I2 is a solid
Noble Gas
nonmetal elements in group 18, each has a full set of valance electrons and are nonreactive
Malleable
ability to be pounded into sheets
Ductile
ability to be pulled into a wire
Conductor
a substance that allows for the flow of heat and electricity
Insulator
a substance that does not allow for the flow of heat and electricity