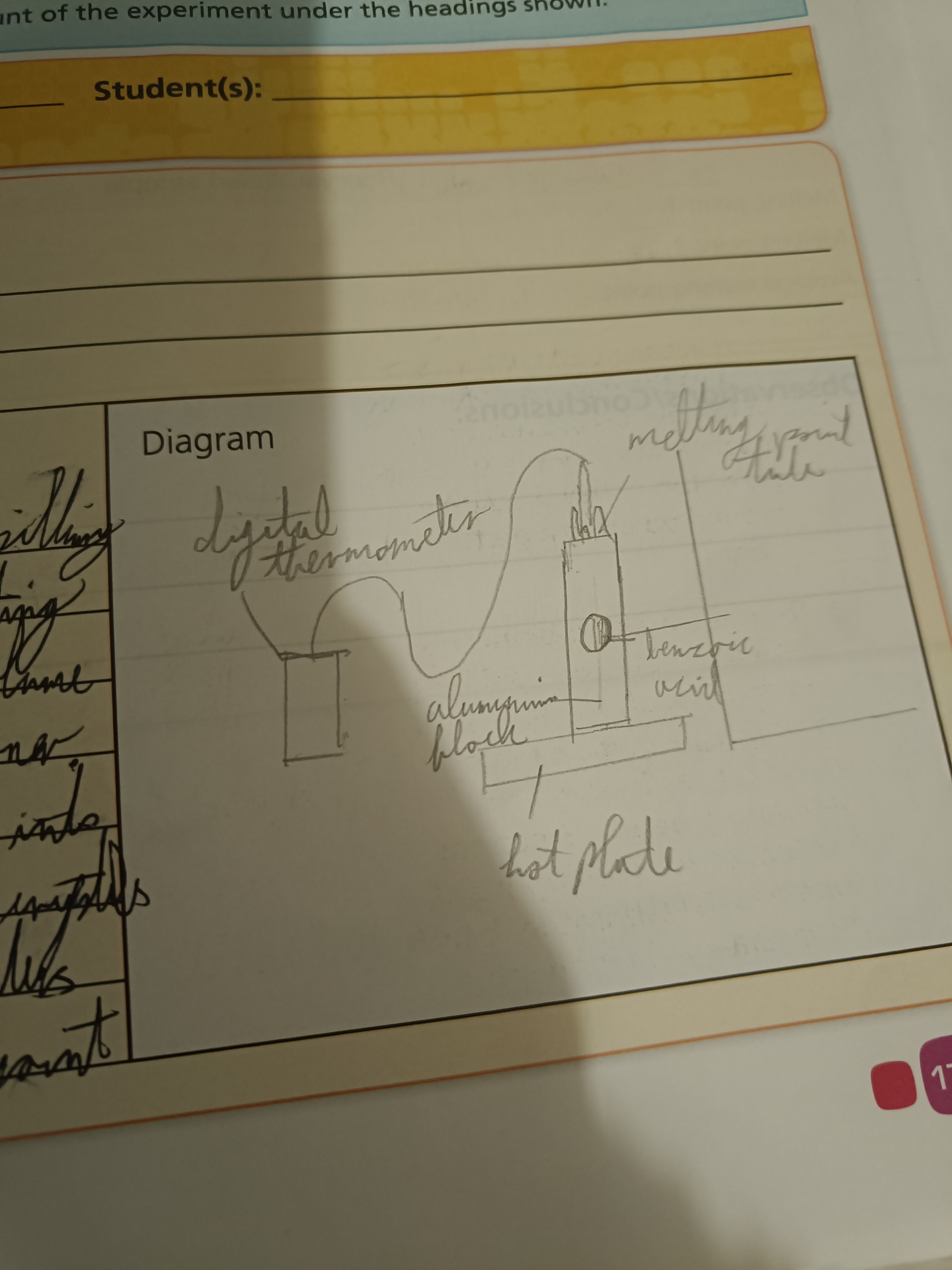
Measure The Boiling Point of Benzoic Acid
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Last updated 9:16 PM on 4/16/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
Step 1
Seal end of a capillary tube by rotating it in the blue flame of a Bunsen burner.
2
New cards
Step 2
Rotate the open end into the benzoic acid crystals so that some enters the melting point tube
3
New cards
Step 3
Set up apparatus as shown with the sealed end pointing down
4
New cards
Step 4
Turn on hotplate and observe the crystals until they melt
5
New cards
Step 5
Note the temperature at which the crystals begin to melt
6
New cards
Step 6
Note temperature at which the crystals finish melting
7
New cards
The range achieved from this experiment is what?
The melting point of benzoic acid
8
New cards
The more pure the benzoic acid is the …
Narrower the range of the boiling point
9
New cards
Draw a diagram of this experiment
…