lattice enthalpy
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
Enthalpy of formation definition
enthalpy change when 1 mole of a compound is formed from its elements under standard conditions (298 K and 100 kPa)
is enthalpy of formation exo or endo
can be endothermic or exothermic
Ionisation enthalpy definition
energy required to remove one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous 1+ ions
is ionisation energy endo or exo?
always endothermic as energy is need to overcome the attraction between an electron and the nucleus
ionisation energy equation:
Na (g) → Na+ (g) + e– ΔHieꝋ = +500 kJ mol-1
Enthalpy change of atomisation definition
enthalpy change when 1 mole of gaseous atoms is formed from its element under standard conditions
atomisation endo or exo?
always endothermic as energy is always required to break any bonds between the atoms in the element, to break the element into its gaseous atoms
Since this is always an endothermic process, the enthalpy change will always have a positivevalue
equation for atomisation:
Na (s) → Na (g) ΔHatꝋ = +108 kJ mol -1
Bond enthalpy definition
amount of energy required to break one mole of a specific covalent bond in the gas phase is called the bond dissociation energy
Bond enthalpy is usually treated as a bond breaking process, so it is quoted in data tables as an ____ energy change with positive values
endothermic
bond enthalpy equation:
Cl2 (g) → 2Cl (g) E(Cl-Cl) = +242 kJ mol -1
Lattice energy definition:
1 mole of an ionic compound is formed from its gaseous ions (under standard conditions)
is it endo or exo?
exothermic, as when ions are combined to form an ionic solid lattice there is an extremely large release of energy
Since this is an exothermic process, the enthalpy change will have a negative value
Because of the huge release in energy when the gaseous ions combine, the value will be a very large negative value
The large negative value of ΔHlattꝋ suggests that the ionic compound is much more stable than its gaseous ions
This is due to the strong electrostatic forces of attraction between the oppositely charged ions in the solid lattice
Since there are no electrostatic forces of attraction between the ions in the gas phase, the gaseous ions are less stable than the ions in the ionic lattice
The more exothermic the value is, the stronger the ionic bonds within the lattice are
lattice enthalpy:
Na+(g) + Cl-(g) → NaCl (s) ΔHlattꝋ = -776 kJ mol -1
enthalpy of neutralisation definition + equation:
enthalpy change when 1 mol of water is formed in reaction between an acid and alkali under standard condition
½ H2SO4 + NaOH —> ½ Na2SO4 + H20
electron affinity def + equation:
first electron affinity= enthalpy change when each atom in one mole of geaousous atoms gains on electron to form 1 mole of geaous 1- ions
2nd= each ion in one mol of geasous 1- ions gains one electron to form on mole of geaous 2- ions
O(g) + e- —> O-(g)
electron affinity:
1st= exo
2nd= endo
hydration enthalpy:
enthalpy change when 1 mol of gaesous ions become hydrated
exo
Mg 2+ (g) + aq —> Mg 2+ (aq)
enthalpy of solu.
one mole of ionic solid dissolves in an amount of water large enough so that the dissolved ions are well seperated and do not interact with each other
exo/endo
MgCl2(s) + aq —> Mg 2+(aq) + 2Cl- (aq)
enthalpy of fusion:
enthalpy change when 1 mol of a solid is turned into a liquid
endo
Mg(s) —>Mg(l)
born-haber cycle left side:

atoms in normal states: Na(s) + ½ Cl2
The enthalpy of atomisation of sodium is
Na (s) → Na (g) ΔHatꝋ = +108 kJ mol -1
The enthalpy of atomisation of chlorine is
½Cl2 (g) → Cl (g) ΔHatꝋ = +121 kJ mol -1
right side:
The sodium ion loses an electron, so this energy change is the first ionisation energy for sodium
Na (g) → Na+ (g) + e– ΔHieꝋ = +500 kJ mol-1
The change is endothermic so the direction continues upwards
The chlorine atom gains an electron, so this is electron affinity
Cl (g) + e– → Cl- (g) ΔHeaꝋ = -364 kJ mol-1
The exothermic change means this is downwards
3:
The enthalpy of formation of sodium chloride is added at the bottom of the diagram
Na(s) + ½Cl2 (g) → NaCl (s) ΔHfꝋ = -411 kJ mol -1
This is an exothermic change for sodium chloride so the arrow points downwards
Enthalpy of formation can be exothermic or endothermic, so you may need to show it above the elements ( and displaced to the right) for a endothermic change
The final change is lattice enthalpy, which is usually shown a formation. For sodium chloride the equation is
Na+(g) + Cl-(g) → NaCl (s) ΔHlattꝋ
how to work out overall change:
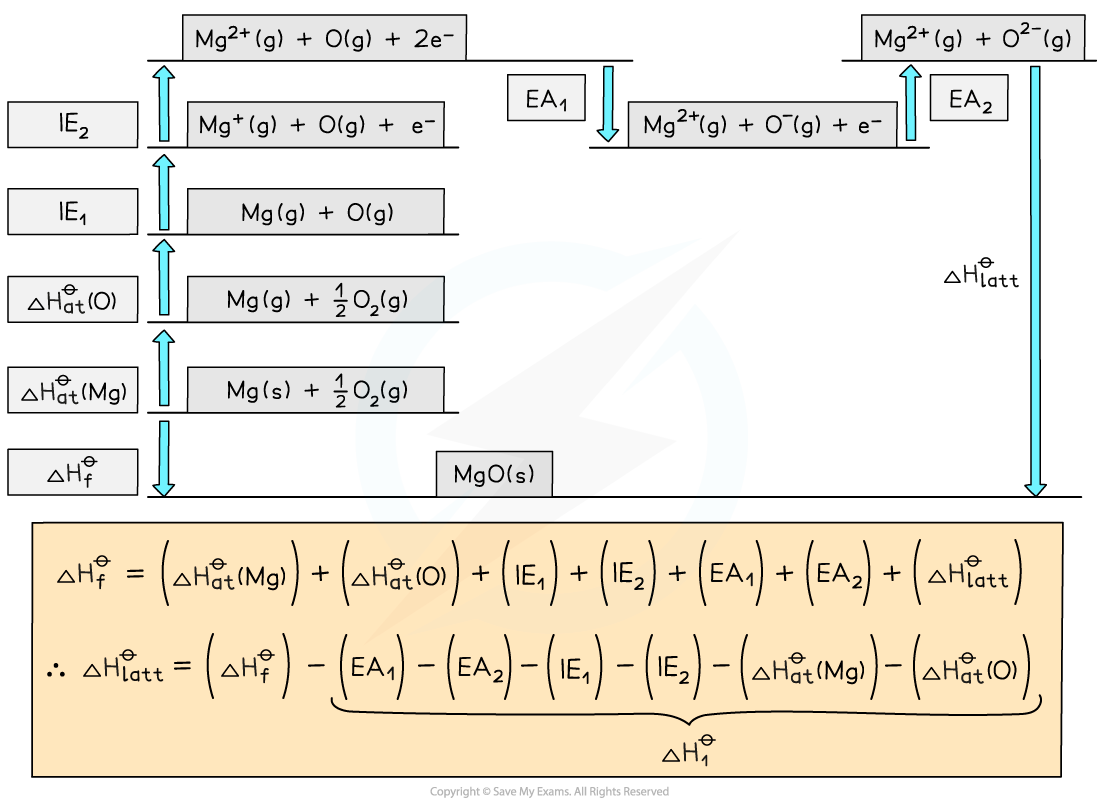
standard enthalpy change of solution (ΔHsol) is the enthalpy change when ____
1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution
standard enthalpy change of hydration (ΔHhyd) is the enthalpy change when _______
1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute solution
When an ionic solid dissolves in water, positive and negative ions are formed
Water is a polar molecule with a δ- oxygen (O) atom and δ+ hydrogen (H) atoms which will form ion-dipole attractions with the ions present in the solution
The oxygen atom in water will be attracted to the positive ions and the hydrogen atoms will be attracted to the negative ions


According to Hess’s law, the enthalpy change for both routes is the same, such that:
ΔHhydꝋ = ΔHlattꝋ + ΔHsolꝋ


What is the affect of increasing ionic radius on lattice energy?
The lattice energy becomes less exothermic as the ionic radius of the ions increases
why?
because the charge on the ions is more spread out over the ion when the ions are larger
The ions are also further apart from each other in the lattice
The attraction between ions is between the centres of the ions involved, so the bigger the ions the bigger the distance between the centre of the ions
Therefore, the electrostatic forces of attraction between the oppositely charged ions in the lattice are weaker
For example, the lattice energy of caesium fluoride (CsF) is less exothermic than the lattice energy of potassium fluoride (KF)
Since both compounds contain a fluoride (F-) ion, the difference in lattice energy must be due to the caesium (Cs+) ion in CsF and potassium (K+) ion in KF
Potassium is a Group 1 and Period 4 element
Caesium is a Group 1 and Period 6 element
This means that the Cs+ ion is larger than the K+ ion
There are weaker electrostatic forces of attraction between the Cs+ and F- ions compared to K+ and F- ions
As a result, the lattice energy of CsF is less exothermic than that of KF

What happens to lattice energy as ionic charge increases?
lattice energy gets more exothermic as the ionic charge of the ions increases
why?
greater the ionic charge, the higher the charge density
This results in stronger electrostatic attraction between the oppositely charged ions in the lattice
As a result, the lattice energy is more exothermic
For example, the lattice energy of calcium oxide (CaO) is more exothermic than the lattice energy of potassium chloride (KCl)
Calcium oxide is an ionic compound which consists of calcium (Ca2+) and oxide (O2-) ions
Potassium chloride is formed from potassium (K+) and chloride (Cl-) ions
The ions in calcium oxide have a greater ionic charge than the ions in potassium chloride
This means that the electrostatic forces of attraction are stronger between the Ca2+ and O2-compared to the forces between K+ and Cl-
Therefore, the lattice energy of calcium oxide is more exothermic, as more energy is released upon its formation from its gaseous ions
Ca2+ and O2- are also smaller ions than K+ and Cl-, so this also adds to the value for the lattice energy being more exothermic
what 2 key factors affect lattice enthalpy?
charge and radius of the ions that make up the crystalline lattice
what factors affect the enthalpy of hydration?
amount that the ions are attracted to the water molecules
The factors which affect this attraction are the ionic charge and radius
If ionic radius decreases what happens to enthalpy of hydration?
ΔHhydꝋ becomes more exothermic with decreasing ionic radii
why?
Smaller ions have a greater charge density resulting in stronger ion-dipole attractions between the water molecules and the ions in the solution
Therefore, more energy is released when they become hydrated and ΔHhydꝋ becomes more exothermic
e.g.
For example, the ΔHhydꝋ of magnesium sulfate (MgSO4) is more exothermic than the ΔHhydꝋ of barium sulfate (BaSO4)
Since both compounds contain a sulfate (SO42-) ion, the difference in ΔHhydꝋ must be due to the magnesium (Mg2+) ion in MgSO4 and barium (Ba2+) ion in BaSO4
Magnesium is a Group 2 and Period 3 element
Barium is a Group 2 and Period 6 element
This means that the Mg2+ ion is smaller than the Ba2+ ion
The attraction is therefore much stronger for the Mg2+ ion
As a result, the standard enthalpy of hydration of MgSO4 is more exothermic than that of BaSO4
Ionionic charge what is the effect on enthalpy of hydration?
ΔHhydꝋ is more exothermic for ions with larger ionic charges
why?
Ions with large ionic charges have a greater charge density resulting in stronger ion-dipole attractions between the water molecules and the ions in the solution
Therefore, more energy is released when they become hydrated and ΔHhydꝋbecomes more exothermic
e.g.
ΔHhydꝋ of calcium oxide (CaO) is more exothermic than the ΔHhydꝋof potassium chloride (KCl)

Calcium oxide is an ionic compound that consists of calcium (Ca2+) and oxide (O2-) ions
Potassium chloride is formed from potassium (K+) and chloride (Cl-) ions
Both of the ions in calcium oxide have a greater ionic charge than the ions in potassium chloride
This means that the attractions are stronger between the water molecules and Ca2+ and O2-ions upon hydration of CaO
The attractions are weaker between the water molecules and K+ and Cl- ions upon hydration of KCl
Therefore, the ΔHhydꝋ of calcium oxide is more exothermic as more energy is released upon its hydration
The entropy (S) of a given system _____
is the number of possible arrangements of the particles and their energy in a given system
In other words, it is a measure of how disordered or chaotic a system is
When a system becomes more disordered, its entropy will _____
increase
An increase in entropy means that the system becomes _______
energetically more stable
during the thermal decomposition of calcium carbonate (CaCO3): CaCO3(s) → CaO(s) + CO2(g)
what happens to the entropy + why?
increase
a gas molecule (CO2) is formed
The CO2 gas molecule is more disordered than the solid reactant (CaCO3), as it is constantly moving around
As a result, the system has become more disordered and there is an increase in entropy
the system with the higher entropy will be ______
energetically favourable (as the energy of the system is more spread out when it is in a disordered state)

feasibility takes no account of the _______
rate of reaction and states only what is possible, not what actually happens. A feasible reaction might be incredibly slow, such as the rusting of iron
What is the unit for entropy?
J K-1 mol-1
equation to calculate the standard entropy change of a system is:
ΔSꝋ = ΣSproductsꝋ - ΣSreactantsꝋ
where Σ = sum of
entropies (S) of the reactants and products
Gibbs equation:
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
When ΔGꝋ is negative, the reaction is ______ and likely to occur
feasible
other equation to work out gibbs:
ΔGꝋ = ΣΔGproductsꝋ - ΣΔGreactantsꝋ
While ∆G can be used to determine the feasibility of a reaction, it does not take into account the ______ of the reaction i.e. rate of reaction
There might be a large energy barrier (Ea) which the reacting species have to overcome before a reaction can occur
Some reactions are feasible since ∆G is negative, but kinetically not feasible since it just occurs too slowly
Such reactions are feasible but very slow
kinetics