chem161, unit 5
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
When You Don’t Need to Draw Every Resonance Structure
You can skip them when all resonance forms are equivalent (same atoms and same charge positions, just mirrored or rotated).
In that case, draw a single resonance hybrid with delocalized electrons using dotted bonds or shared electron pairs
Example:
I₃⁻:
Two equivalent resonance forms (I=I–I⁻ and I⁻–I=I).
The real structure is [:I···I···I:]⁻ — linear, with both I–I bonds equal and electrons delocalized in the center.
Key idea:
If all resonance forms are identical by symmetry, one hybrid structure accurately represents them all.
How does electronegativity determine bond type?
0-0.4 —> pure covalent
0.5-1.7 —> polar covalent
1.7-ect —> Ionic
How to determine electronegativity using periodic table?
non-metal + itself —> pure covalent
non-metal + another non-metal —> polar covalent
non-metal + metal —> Ionic
Note
If the bond is covalent you can check if it is polar or pure covelent via the geometry of its strucutre
(if the electronegativity cancels on geometrically then it is just pure covalent and not polar)
Valence rule for bonding(is it absolute?)
the valence rule is that elements want to have all 8 of their bonds filled
(we can figure out if all bonds are filled if its formal chage is 0)
this rule is not absolute.. why?
→ Less electronegative atoms will be forced into the center of a molecule which causes situations where they could have too many electrons(expanded octet)
expanded octet rule
central atoms in molecules can have more than 8 electrons if..
they are in period 3 or below (period = row)
formal charge reasoning
Formal charge helps us figure out how the electrons are distributed in a molecule compared to how they’d be in neutral atoms.
It tells us if an atom has gained or lost electron control when forming bonds.
We use it to find the most stable (lowest-energy —> closest to a total 0 formal charge) Lewis structure.
formal charge equation
formal charge = (Valence) - (bonds) - (lone pairs)
Note —> lone pairs do need to have 2 electrons…
Best resonance structure
octet rule obeyed
more covalent bonds
less formal charges(closer to zero and less formal charges)
negative charges are on more electronegative atoms
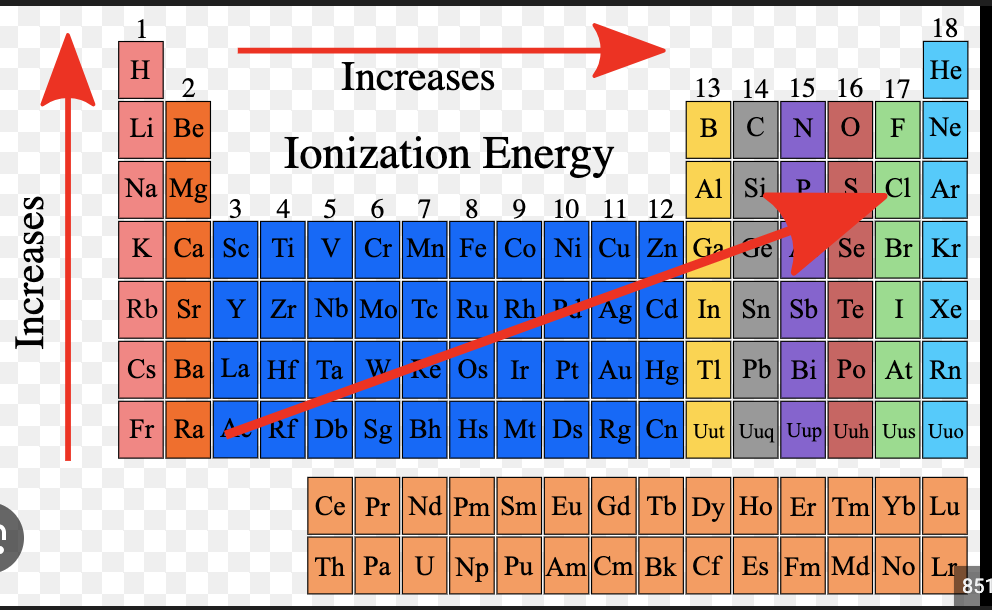
electronegativity trend
Ionization energy
energy required to remove an electron from an atoms..
skeletal structure
corners are carbon
lines are bonds between carbon
the number of bonds of hydrogen on each carbon is based on the number of bonds needed to make a carbon have 4 bonds
octet rule
atoms tend to have the same number of bonds as the number of valence electrons they need this happens since an atom with a full octet is more stable
(so a carbon wants to have 4 bonds in order to get the 8 extra valence electrons to fill up its octet)
molecular polarity(between 2 atoms, and for molecules in general)
Dipole moment (μ) = measure of charge separation due to electronegativity difference.
Steps:
Compare electronegativities → bigger ΔEN → more polar
Draw arrow toward more electronegative atom (δ–)
Optional: μ = Q × r
Molecules(need geometric visual): Add bond dipoles as vectors → net dipole
Symmetric → cancel → μ = 0
Asymmetric → add → μ > 0
Examples: H–Cl (polar), CO₂ (nonpolar), H₂O (polar)
us