energy changes in equations 4.3 chemistry
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
law of conservation of energy
energy cannot be created or destroyed, but can be changed
to break bonds is to
release stored chemical energy
exothermic reactions
hot
energy is released by system to surroundings
endothermic reactions
cold
heat absorbed by system from surroundings
exothermic equations
negative DELTA H
kJ leaves the system
CH4+O2→Co2+H2O+300kJ
endothermic equations
gain energy, kJ on reactant side
CO2+H2O+kJ→O2+C6H12O6
DELTA H positive
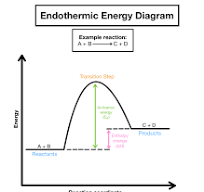
endothermic energy diagram
exothermic energy diagram
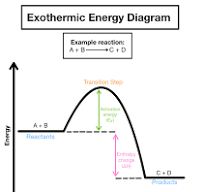
what is shown in Exothermic energy diagram?
reactants have more stored chemical energy than products
energy is released
feals hot
what is shown in endothermic energy diagrams>
products have more stored chemical energy than reactants
energy is absorbed during reaction
cold