Chapter 19 - hormonal regulation of metabolism
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What is metabolism?
Sum of all chemical reactions, including catabolism (breakdown) and anabolism (synthesis).
What measures resting metabolism?
Basal Metabolic Rate (BMR).
What are the three main energy reserves in the body? The body has three primary types of molecules that provide “reserves” of essential building blocks:
Glycogen (liver & muscle), triglycerides (adipose, liver, muscle), protein (not stored, excess → fat/urea).
Which hormones are anabolic vs catabolic?
Anabolic: insulin, sex steroids. Catabolic: glucagon, epinephrine, glucocorticoids.
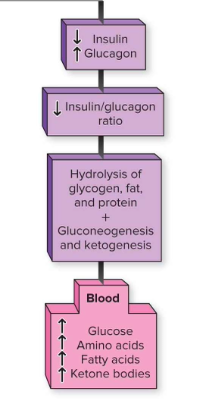
How does metabolism shift after a meal?
↑ Insulin, ↓ Glucagon → anabolism (store glycogen, fat, protein synthesis).
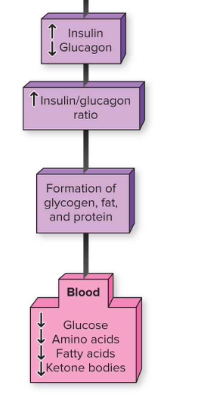
How does metabolism shift during fasting?
↓ Insulin, ↑ Glucagon → catabolism (breakdown glycogen, fat, protein).
What hormone from the intestines stimulates insulin release after a meal?
GLP-1 (binds GPCR in β-cells → cAMP → ↑ insulin release).
What happens to blood nutrient levels during fasting?
Increase (↑ glucose, amino acids, fatty acids, ketones)
Why is glucose homeostasis critical?
Hyperglycemia → kidney failure, retinopathy, coma, death
Hypoglycemia → brain damage, seizures, autonomic dysfunction
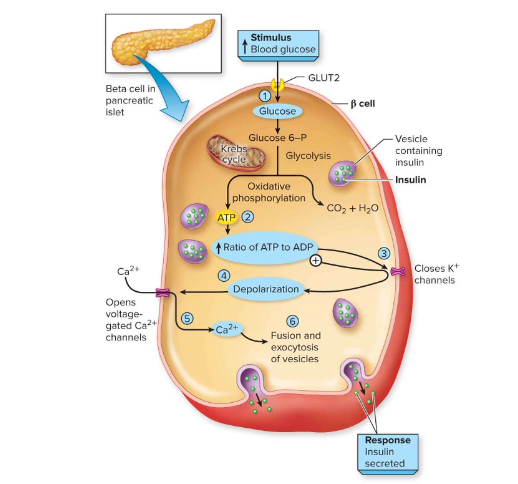
How is insulin secreted from β-cells?
Glucose → ↑ ATP → closes ATP-K+ channels → depolarization → opens Ca²⁺ channels → Ca²⁺ influx → insulin exocytosis.
Fasting plasma glucose homeostasis, when we eat and drink
between 65-105 mg/dL, spike to ~140-150 or more
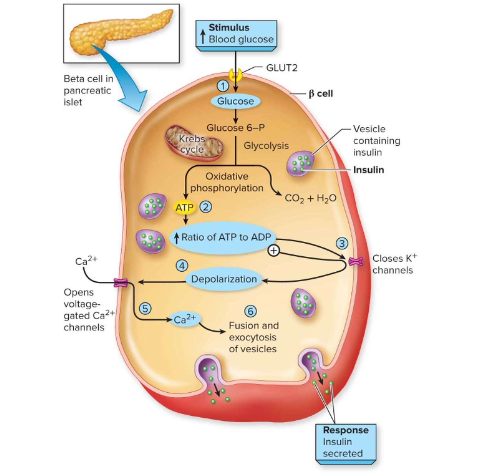
Insulin release is stimulated by absorption of glucose and amino acids after
consuming food or drink: describe the 6 steps
(1) Elevated glucose levels lead to (2) an increase in intracellular ATP, which leads to (3) closing ATP-gated K+ channels. Next, (4) depolarization of the cell opens voltage-gated Ca2+ channels, which (5) allows calcium ion influx. This enables (6) exocytosis of vesicles containing insulin, which travels through the bloodstream and has widespread effects.
What are the main actions of insulin?
Promotes glucose uptake (GLUT-4), glycogen storage, fat storage, protein synthesis. Inhibits gluconeogenesis & lipolysis.
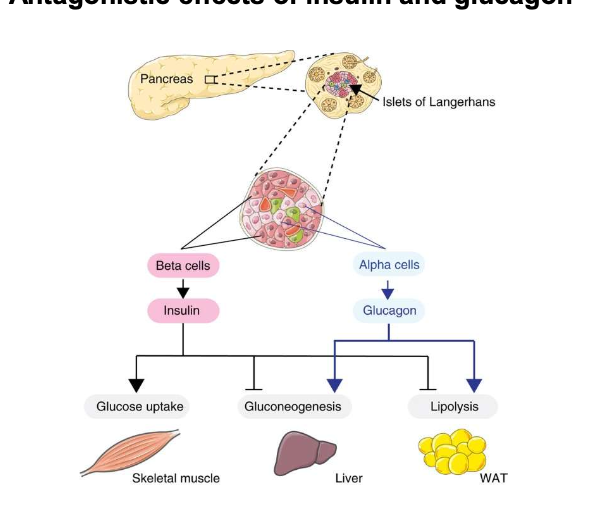
Which pancreatic cells secrete insulin vs glucagon?
β-cells → insulin; α-cells → glucagon.
What are the main effects of glucagon on metabolism?
↑ Glycogen breakdown, gluconeogenesis, lipolysis; ↑ ketone production.
What are glucagon’s fasting effects?
Liver: glycogen breakdown, gluconeogenesis, ketone release
Adipose: lipolysis → fatty acids
Skeletal Muscle: protein breakdown → amino acids
Key differences between Type 1 and Type 2 diabetes?
Type 1: autoimmune, beta cell destruction, ↓ insulin, early onset
Type 2: insulin resistance + ↓ beta cells, later onset, obesity-linked
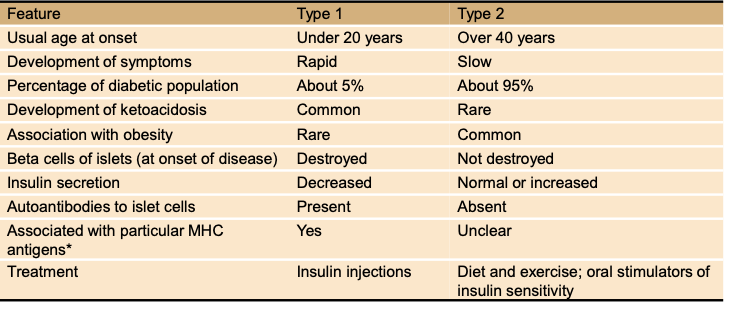
What % of diabetics are Type 1 vs Type 2?
Type 1: ~5%. Type 2: ~95%.
Development of symptoms: Type 1 vs Type 2?
Type 1: rapid. Type 2: slow.
Usual age at onset for Type 1 vs Type 2 diabetes?
Type 1: under 20 years. Type 2: over 40 years (but increasingly younger with obesity).
How do treatments differ between Type 1 and Type 2 diabetes?
Type 1 = insulin injections.
Type 2 = diet/exercise + oral drugs (sometimes insulin).
Shared complications? Type 1 and 2 diabetes
Chronic hyperglycemia, kidney failure, cardiovascular disease, ketoacidosis, blindness.
What does HbA1c measure?
% glycosylated hemoglobin → reflects average blood glucose over 120 days.
Why is it useful?(HbA1c)
Diagnoses prediabetes & diabetes (but less predictive in some populations).
Additional benefits/risks? Semaglutide
Lowers weight, BP, cholesterol. Risks: GI issues, pancreatitis, possible thyroid cancer.
What is Semaglutide and how does it work?
GLP-1 receptor agonist(mimics GLP-1); enhances insulin release (β-cells), inhibits glucagon release (α-cells), delays gastric emptying, and suppresses appetite → lowers blood glucose and HbA1c.
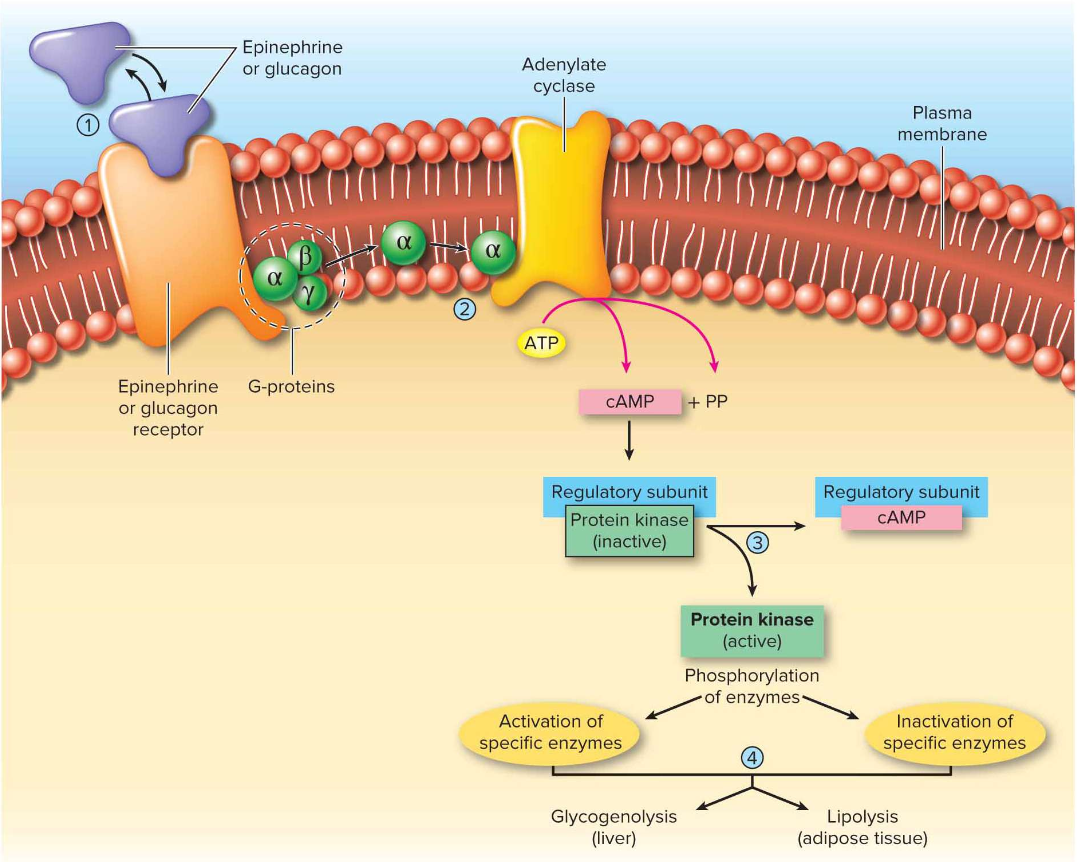
How do glucagon, epinephrine, and norepinephrine signal to increase metabolism?
Via GPCR → G-protein → cAMP 2nd messenger → activates kinases → glycogenolysis (liver) & lipolysis (adipose).
What is the role of epinephrine and norepinephrine in fight-or-flight?
Increase glucose & fatty acid release for energy, vasoconstrict digestive/kidney vessels, bronchodilate lungs, supply extra fuel to heart, brain, and skeletal muscle.
Which organs release glucagon vs epinephrine/norepinephrine?
Glucagon = pancreatic α-cells; Epinephrine & NE = adrenal medulla.
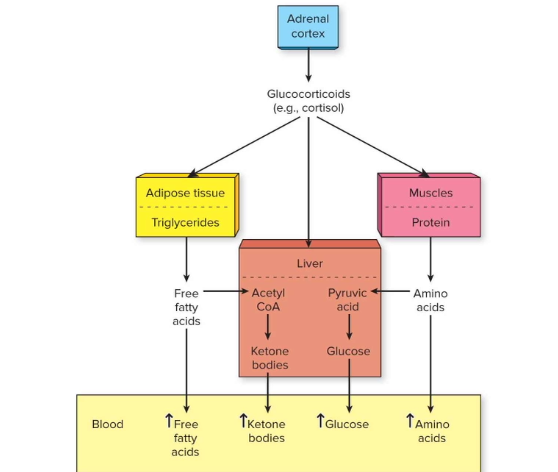
Where are glucocorticoids like cortisol produced, and what stimulates their release?
Adrenal cortex; CRH → ACTH → cortisol pathway.
How do cortisol’s metabolic effects compare to epinephrine/norepinephrine?
Similar (↑ glucose & fatty acids) but also ↑ ketone body release (liver) and ↑ amino acid release (muscle).
What are the main fuel sources released into blood under cortisol’s influence?
Glucose, free fatty acids, ketone bodies, and amino acids.
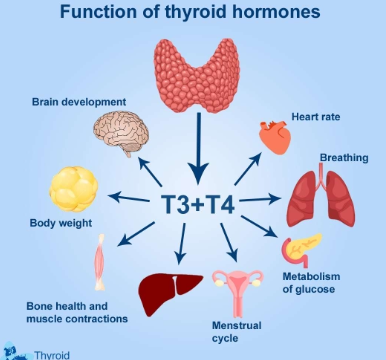
Functions of thyroid hormones?
• Acts on most cells in the body to increase rates of cellular respiration
• Increases production of metabolic heat
• Sets basal metabolic rate (BMR)
• Regulates balance between anabolism and catabolism
What are the anabolic functions of GH?
Stimulates bone/cartilage growth, promotes amino acid uptake and protein synthesis
What are the catabolic functions of GH?
Promotes lipolysis (fat breakdown), reduces glucose use for energy.
What role does GH play in adults?
Important for bone remodeling by regulating activity of osteoblasts and osteoclasts.
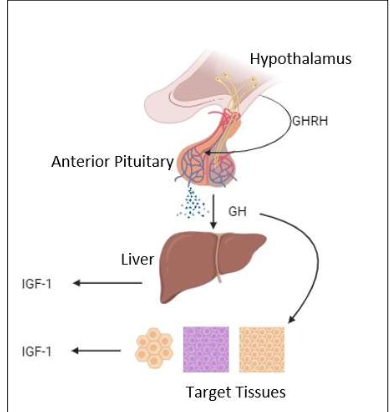
How is GH released and what hormone mediates many of its effects?
GH released from anterior pituitary (via GHRH); effects mediated by IGF-1 from the liver.
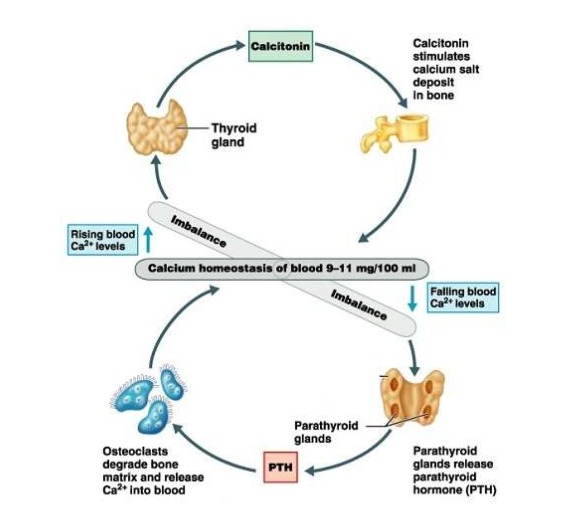
What happens when plasma calcium levels rise?
Calcitonin released from thyroid → stimulates osteoblasts → Ca²⁺ deposited in bone → lowers blood Ca²⁺.
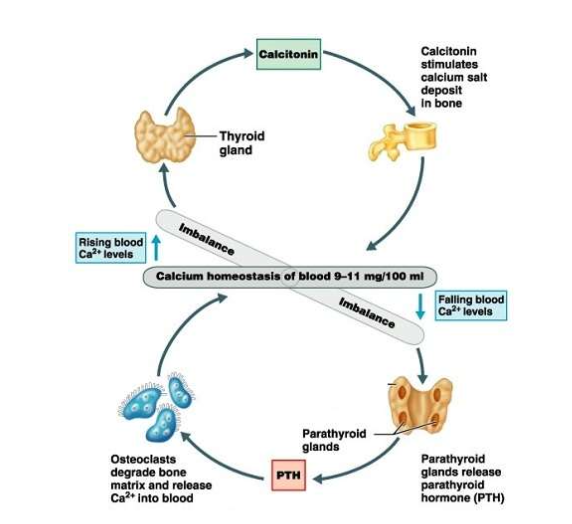
What happens when plasma calcium levels fall?
PTH released from parathyroid → stimulates osteoclasts → bone breakdown & Ca²⁺ release → kidneys reabsorb more Ca²⁺ → raises blood Ca²⁺.
How do calcitonin and PTH work together?
They act antagonistically to maintain calcium homeostasis (9–11 mg/100 ml)
Which hormones affect bone physiology?
Estrogen, testosterone, insulin, thyroid hormones, vitamin D, PTH, calcitonin.
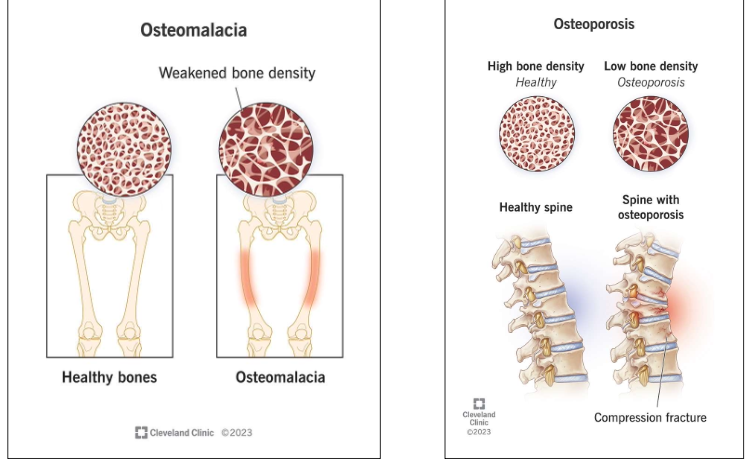
What diseases result from imbalance?
Osteomalacia (rickets, poor mineralization) and osteoporosis (low bone density, fractures).
What is osteoporosis and who is most at risk?
Reduction in bone density and bones that are more prone to fracture. Men are less prone to osteoporosis because they produce testosterone their whole lives, while women stop producing estrogen at menopause, or in some cases women taking estrogen blocking drugs as a treatment for breast cancer
What is osteomalacia, and what causes it?
rickets, a disease associated with lack of calcium phosphate deposition in bones.
Causes include dietary insufficiency, or underlying disease (Crohn’s disease, cancer, liver disease)