cell migration
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
49 Terms
cell polarization and migration
defines the front and the rear, allowing cells to move in one direction
guided by the nucleus, golgi apparatus, and MTs → i.e., asymmetrical emanation of MTs from the centrosome pulls the nucleus behind them
defines the direction of polarization in most mesenchymal cells via nuclear translocation and MT polarization
protrusion or microspikes
refers to filopodia, lamellipodia, invadopodia, and blebbing
filopodia
common in migrating growth cones of neurons and fibroblasts
one dimensional, long bundles of parallel actin → "thin “finger-like” projections
contains receptors, allowing response to the immediate, surrounding cellular environment
function of filopodia
acts as cellular “antennae”, probing the cell microenvironment
constructs cell-cell adhesions and guides growing dendrites to chemoattractants
lamellipodia
common in epithelial cells, fibroblasts, and neurons → wound-healing
two-dimensional, mesh-like network of branched actin that stretch out to the cell periphery
lamellipodia machinery
actin filaments for rapid movement
MTs and IFs restricted to the immediate area around the nucleus
dependent on the ARP2/3 complex
the role of ARP2/3 complex in lamellipodia cell motility
anchors minus ends to other actin filaments, creating actin mesh → plus ends of actin filaments orient towards the leading edge of the cell
facilitates the nucleation of new actin polymers at a 70 degree angle to the existing actin polymer
when absent → there is still some cell motility governed by filopodia, not lamellipodia
function of lamellipodia
cell motility → generates pushing forces, driving the cell forward
directs motility by: controlling cell adhesion and giving the cell a polarized structure, acting as an “internal compass”
the role of cofilin
disassembles actin filaments
has a higher affinity for ADP-actin
localizes to the rear of the leading edge → ensures actin polymerization is to the leading edge only
allows continuous unidirectional movement (i.e., that enough actin monomers are available locally for growth)
invadopodia
often found in metastatic cancer
three-dimensional actin, rich in protrusions that effectively penetrate tissue barriers → “invasive feet”
invadopodia machinery
starts from localized loss of the actin cortex → blebbing to form an immature filopodia-like structure
maturation results in degradation of the surrounding ECM → creates a point of entry for migration into other tissues
blebbing
protrusion of the membrane due to loss of the actin cortex/formation of an immature spine
membrane blebbing
blebs form when the PM detaches from the cytoskeleton/underlying actin cortex
contraction of myosin in the absence of an opposing actin cortex → membrane protrusion
reassembly of the actin cortex, establishing a new cellular position
actin polymerization
drives PM protrusion
the leading edge results from actin contraction at the rear, pushing the PM forward
movement of cells across a solid substratum
lamellipodia at the leading edge
localized contraction and actin polymerization at the rear pushes lamellipodia forward → gives room for the formation of new focal adhesions
growth cones
guides a developing axon to its synaptic target
involves actin and MT cytoskeletons that steer the axon → directional migration
growth cone dynamics
extension of the filopodia, contacting an adhesive substance via receptors on the actin cytoskeleton → some depolymerization at the minus end, no polymerization at the plus end
vesicle fusion at the PM, allowing space for actin filament polymerization → supports the growth of filopodia at the plus end/leading edge
actin polymerization pushes filopodia forward to extend and contract
advancement of MTs from the core
entry of cytoplasm/organelles to MTs/motor proteins → advancement of the axon
invadopodia in breast cancer
stage 0 → no lamellipodia
stage 1 → initial membrane relaxation and blebbing, resulting in F-actin polymerization pushing forward and stabilization of branched actin
stage 2a → migration through the ECM
stage 2b → MT and IF movement, pulling along other cellular components
invadopodia formation in a breast cancer cell
where actin is concentrated, laminin is less concentrated
eventually results in a gole, allowing blebbing
myosin contraction and cell adhesion
allows cells to pull themselves forward and promotes the formation of focal adhesions → coupled with de-adhesion at the rear of the cell
myosin links the actin cytoskeleton to the substratum via integrin-mediated adhesion
the role of myosin II
associates with actin filaments at the rear of lamellipodia to pull them to a new position → from perpendicular to parallel to the leading edge
pulls the cell towards the leading edge, maintaining contact with the substratum by leaving focal adhesion contacts intact
premature loss of focal adhesions
allows actin filaments to slip back away from the leading edge following actin polymerization
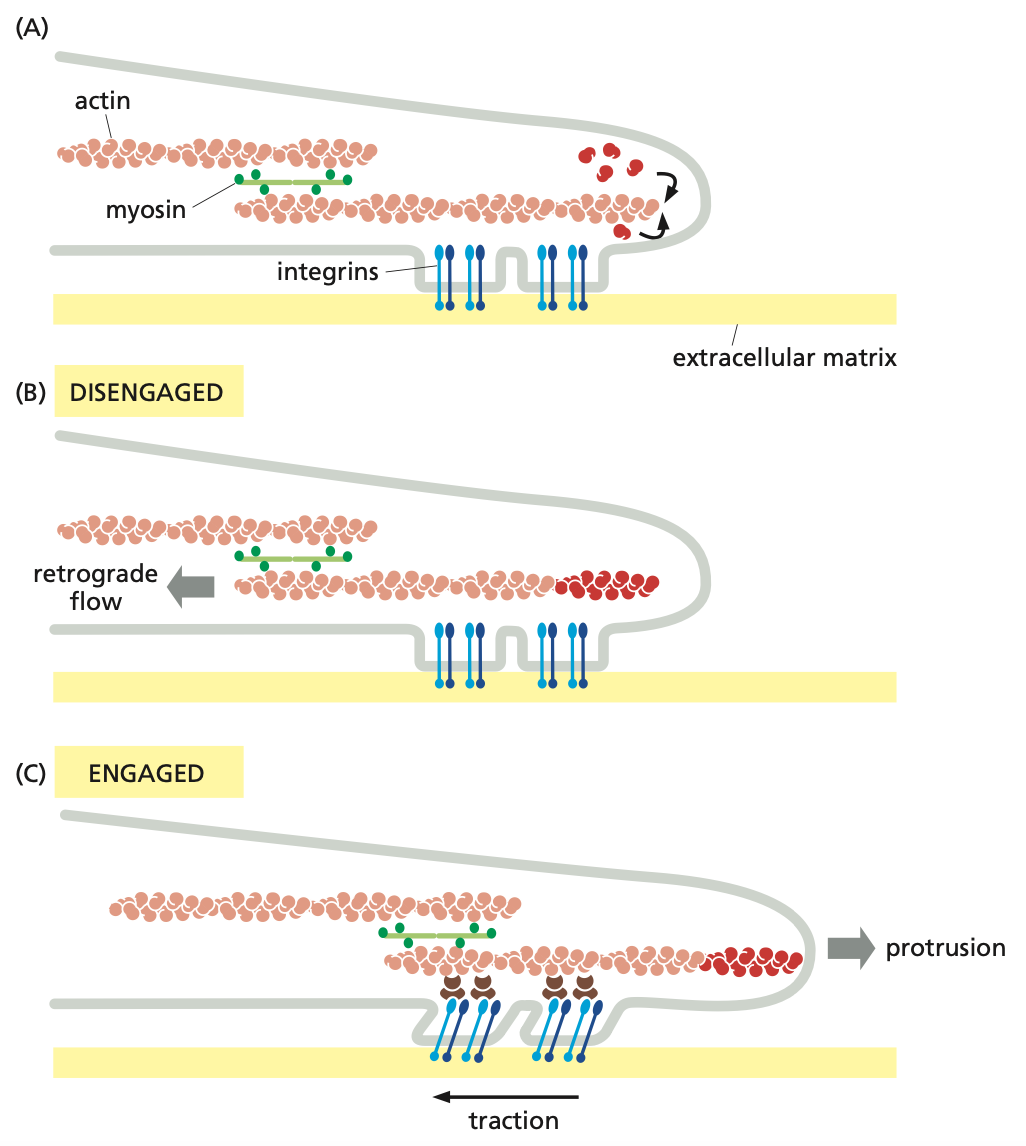
control of cell-substratum adhesion
A: assembly of actin monomers at the leading edge of actin filaments → integrins help form focal adhesions, linking the cell membrane to the substratum
B: no interaction between actin filaments and focal adhesions drives the retraction of the actin filament → drives the actin filament rearward via polymerization pressure at the leading edge and myosin contraction, causing retrograde slippage
C: interaction between actin filaments and focal adhesion drives the contraction of the actin filament → drives the leading edge forward via the transmission of myosin contraction through the focal adhesion, generating traction on the ECM, and new actin polymerization
cell polarization
controlled by members of the Rho family (i.e., CDC42, Rac, and Rho)
must distinguish between the leading and rear edges before migration via polarization of the cytoskeleton
the role of CDC42 in filopodia formation
triggers actin polymerization and bundling through activation on the inner surface of the PM → triggers filopodia formation
activates WASp protein family members (i.e., N-WASp)
stabilizes the open/active N-WASp protein conformation, binding profilin in filopodia and some ARP2/3 in lamellipodia
promotes primarily filamentous actin nucleation, and some branched actin nucleation
lamellipodia formation via Rac
activation triggers actin polymerization in the cell periphery → triggers the formation of sheet-like lamellipodia
activates WASp → activates the ARP2/3 complex in lamellipodia, and some profilin in filopodia
activates filamin and Rho, inhibits myosin II
Rac: the role of filamin
an actin crosslinking protein
stabilizes orthogonal actin networks in lamellipodia
Rac: the role of Rho
induces myosin contraction in the region opposite to the leading edge
only occurs after the lamellipodia is fully formed
Rac: the role of myosin II
inhibits myosin II via activation of PAK and inhibition of MLCK
results in no contraction and stable lamellipodia
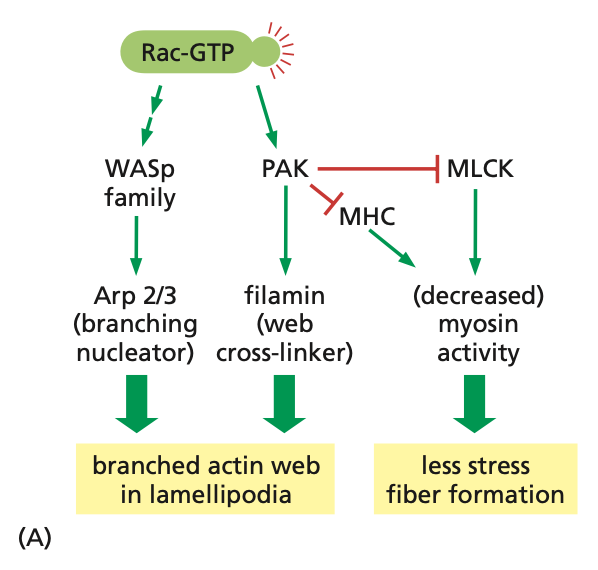
Rac activation pathways
active Rac (Rac-GTP) activates WASp, which activates ARP2/3, giving branched actin in lamellipodia
Rac-GTP also activates PAK, which inhibits MHC/MLCK, resulting in decreased myosin activity/contractility, and less stress fiber (F-actin) formation
RAC-GTP also activates PAK, which activates filamin, resulting in the stabilization of branched actin in lamellipodia
contractile force and migration via Rho
activation promotes myosin activity → bundles of actin filaments/myosin II results in stress fiber formation and integrins cluster into focal adhesions
activation also promotes MLC phosphorylation → inhibits MLC phosphatase, promoting myosin contraction
activates formin and inhibits cofilin
Rho: the role of formin
promotes actin bundling
Rho: the role of cofilin
inhibits cofilin via activation of LIM kinase
blocks actin depolymerization
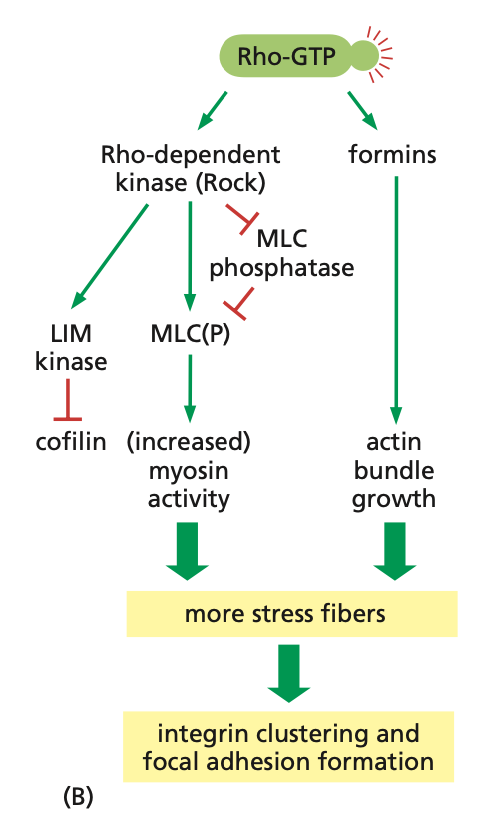
Rho activation pathways
active-Rho (Rho-GTP) activates ROCK, which inhibits MLC phosphatase, deactivating MLC(P), and increasing myosin activity
active ROCK also activates LIM kinase, inhibiting cofilin, resulting in decreased polymerization and actin filaments
Rho-GTP also activates formins, increasing actin bundle growth
→ more stress fiber formation and integrin clustering/focal adhesion formation
chemotaxis
dictates the direction of cell migration
chemotactic signals promoting cell migration towards/away from the signal
ECM adhesion
proteins in the matrix associate with the growth cone
cell-surface adhesion
proteins on the surface of the cell represent signals attracting the growth cone, providing a surface for migration
fasciculation
if there is already a path to follow, the growth cone follows it
chemoattraction
an area where a signaling factor is released, attracting the growth cone towards it
chemorepulsion
an area where a signaling factor is release, repulsing the growth cone away from it
contact inhibition
repulsion occurring when growth cones come into contact with something (i.e., a specific cell type)
the role of chemotaxis
initiates a signaling cascade
involves a chemoattractant binding its GPCR on the migrating cell, activating PI3K and activating RAC
RAC → activates ARP2/3, promoting lamellipodia formation
PI3K → rapidly degraded, cannot diffuse to provide directionality to the newly formed actin mesh
GPCR activation → activates Rho, promoting myosin contraction (i.e., Rac in the leading edge + Rho in the rear → polarity to the cascade)
neutrophil polarization
enables the cell to maintain functional polarity with protrusion at the leading edge and contraction at the back
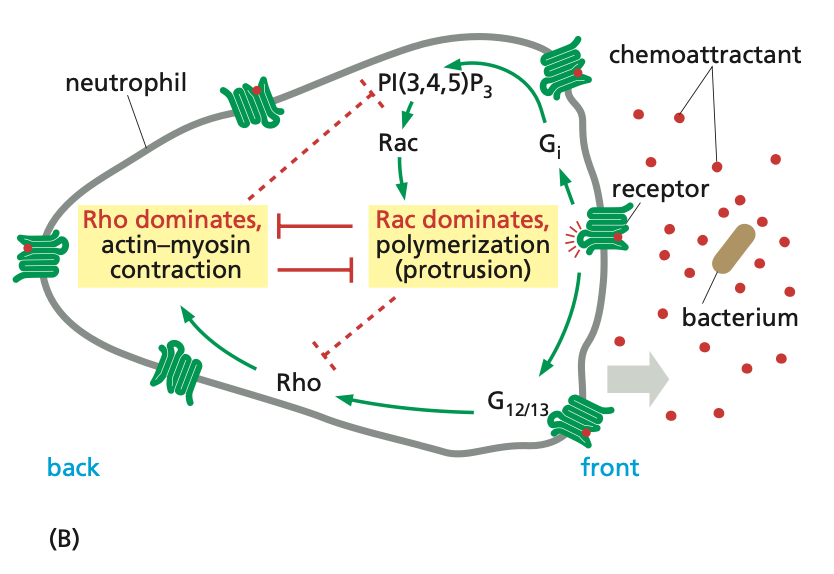
steps to neutrophil polarization
chemoattractant → binds GPCR → activates PI3K and PIP3 → activates Rac → signaling cascade → forms branched actin of lamellipodia and F-actin of filopodia
components of neutrophil polarization
PI3K and PIP3 act locally at the front of the cell → does not diffuse to the back of the cell
GPCR simultaneously activates Rho at the back of the cell, which goes through its signaling cascade, promoting the formation of stress fibers and contraction
Rac and Rho
inhibit each other
gives polarity and direction of migration
putting it all together (1)
Rho-GTP → ROCK → pMLC → local myosin II contraction
CDC42 → WASp → mDia → filamentous actin
Rac1 → WASp → Arp2/3 → actin mesh
putting it all together (2)
Rac-GTP → activates WASp → activates Arp2/3 → promotes the nucleating of branched actin → forms the meshwork of the lamellipodia
AT THE FRONT OF THE LEADING EDGE OF THE CELL: active CDC42 → activates WASp → activates formin → promotes the nucleation and growth of F-actin
AT THE REAR EDGE OF THE CELL: Rho-GTP → activates ROCK → phosphorylated MLC → increases myosin contraction
putting it all together (3)
gradient between Rho-GTP and Rac-GTP maintains a separation
Rho inhibits Rac at the rear of the cell, pushing it to the front of the leading edge of the cell where Rac inhibits Rho, pushing it to the back of the cell → net force for direction of migration