periodic table and hydrogen
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
What is the metallic radius?
Half the distance between the centre of the atoms in the solid state of an element
What is the covalent radius?
Half the internuclear distance of neighbouring atoms of the same element in a molecule
half the distance between the nuclei
What does ‘atomic radii’ consist of?
Metallic and covalent radius
How does atomic radii change across a group and period?
increases in size down a group
decreases in size along a period
What is the first ionisation energy of an element?
The change associated with the removal of the first valence electron at 0K and 1atm from a gaseous atom
What is the difference between ionisation energy and enthalpy?
Ionisation energy is at 0K and 1atm
Ionisation enthalpy ΔHion is at 298K
What is electron pairing energy?
Electron-electron repulsion causes lower than expected ionisation energy
What are the lowest and highest IE values?
Highest = noble gases as filled shells
Lowest = alkali metals - single electrons outside a full shell
What is the first electron affinity EA1?
What is the equation?
The internal energy released when an electron is added to a neutral gas phase atom
X (g) + e- → X- (g)
What is electronegativity?
What is it an indicator of?
The ability of an atom to attract electrons to itself in a molecule
It is an indicator of:
general chemical behaviour
electron distribution in a bond
whether a compound will be ionic or covalent
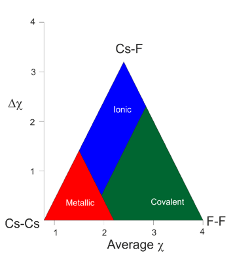
What does the Ketelaar triangle look like?
Do metals conduct electricity when:
solid?
molten?
Yes and yes
Are metals soluble in water?
No
What does melting point and strength depend on in metals?
What does strength of metallic bonding depend on?
Depends on strength of underlying metallic bonding
depends on how many electrons are delocalised or given up into the electron sea
Why is H2 relatively unreactive?
It is non labile, and has high activation energy
3 examples of H2 reacting rapidly?
free radical chain reactions
homolytic dissociation at a metal surface
heterolytic dissociation at a metal surface
3 main electronic processes of hydrogen reactions
losing electron to make H+
gaining electron to make H-
sharing electrons to make covalent molecules
Why is hydrogen able to penetrate metals?
What does it form?
It is able to penetrate metals due to small size of H atom
Forms metallic hydrides

Why do LiH and BeH2 have high melting points?
They have extended lattice structures

Why does diborane and methane have very low melting points?
They are molecular with London dispersion forces

Why do NH3, H2O and HF have fairly low melting points?
They have LDFs and hydrogen bonds
Where in the periodic table are saline hydrides?
How are they formed?
What are the partially positive charges?
Formed by the electropositive elements of groups 1 and 2 (s block)
E𝛿+-H𝛿-
Saline hydrides
Ionic or covalent?
What colour?
High or low melting point?
Ionic
Colourless
High melting points
What is the structure of group 1 MH?
What is the structure of group 2 MH2?
MH = rock salt structure
MH2 = range of structures
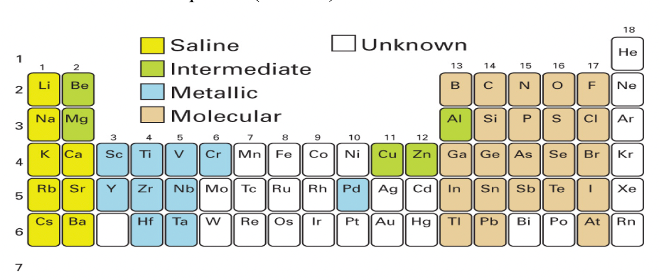
Where in the periodic table are the metallic hydrides?
Which elements form them i.e. which block?
Formed by the d and f block elements
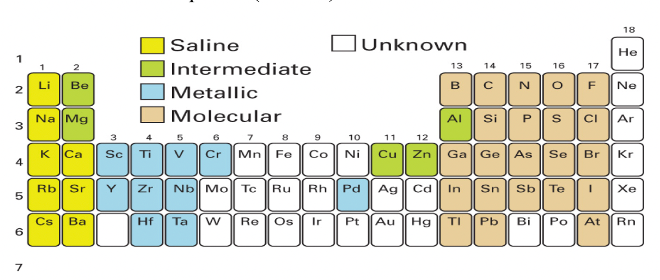
Where in the periodic table are the intermediate hydrides?
What are their properties?
Intermediate between the ionic and molecular hydrides
Be, Mg, Cu, Zn, Al
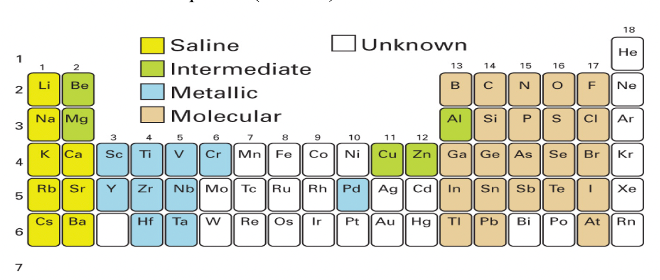
Where in the periodic table are the covalent molecular hydrides?
Which elements form them (which block)?
What are the three types?
Formed by electronegative elements from groups 13-18 (p block)
electron deficient
electron precise
electron rich
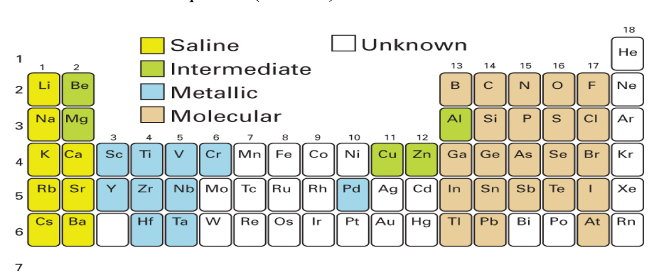
What happens to the bond when an H atom is covalently bonded to an electronegative element?
The bond is polarised
Evidence for hydrogen bonds
Anomalously high boiling points of HF, NH3, and H2O
they can form H bonds in the liquid state
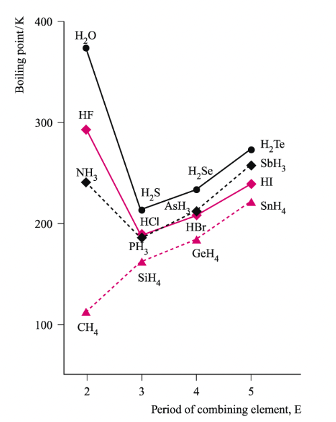
What causes dipole-dipole forces?
Electrostatic attraction between the dipoles
there must be an overall dipole on the molecule
What causes London dispersion forces?
Instantaneous dipoles which induce dipoles in neighbouring molecules