Lysosomes, Mitochondria, and Cellular Processes Overview
1/151
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
152 Terms
Lysosomes
Organelles containing hydrolytic enzymes for digestion
single membrane organelle
uses 50+ hydrolytic enzymes
active at pH=5
De Duve
Scientist who discovered lysosomes.
how were lysosomes discovered
Centrifugation: Technique to separate organelles based on density.
organelle purification
Hydrolytic enzymes
Enzymes that catalyze the breakdown of molecules.
What distinguishes lysosomes from other organelles
lysosomes have an acid phosphatase (a marker enzyme)
what is a marker enzyme
enzyme found in one and only one organelle to serve as a means of identification
3 functions of lysosome
heterophagy
autophagy
endocytosis
Heterophagy
Digestion of external substances by lysosomes.
Autophagy
Self-digestion of cellular components by lysosomes.
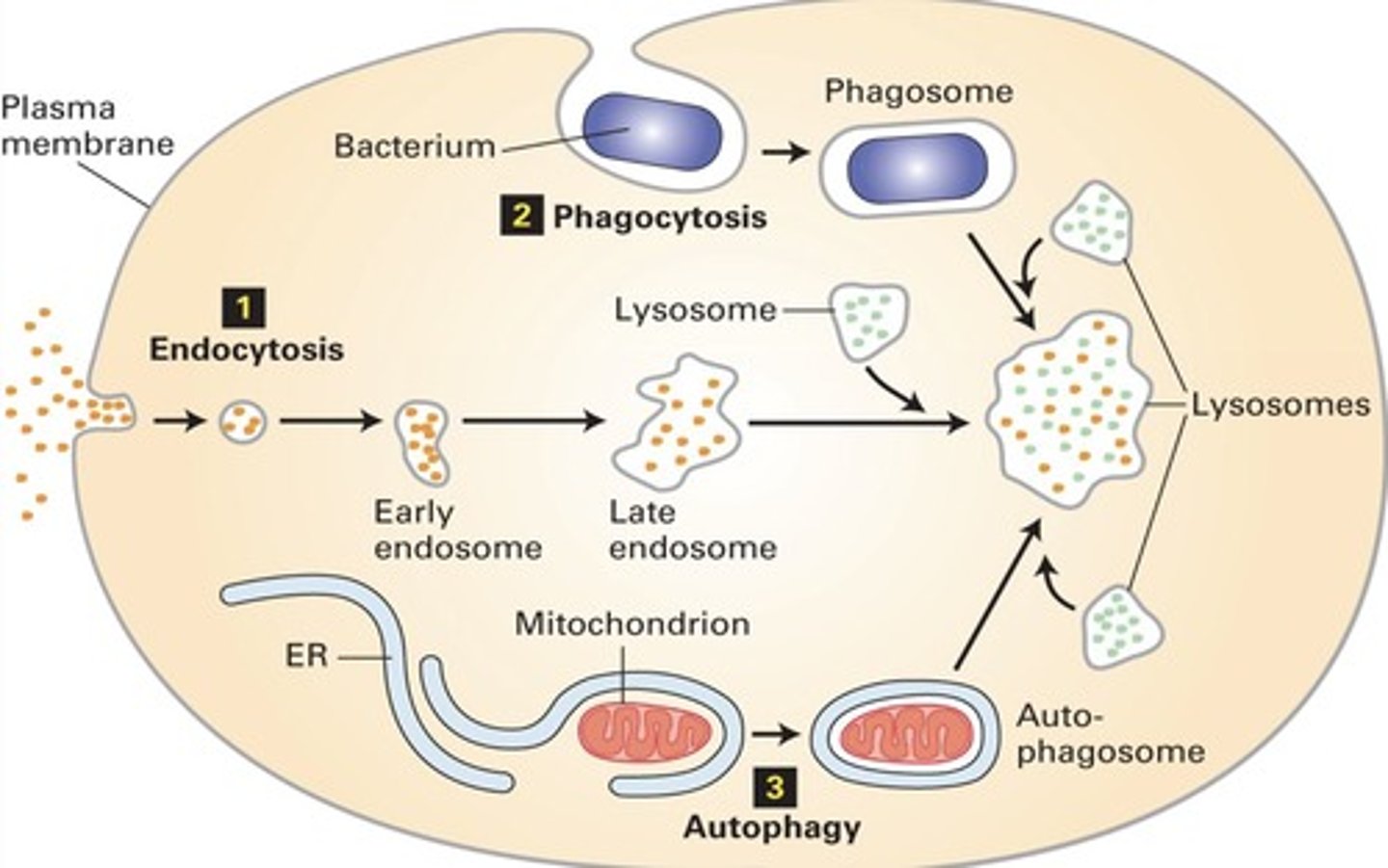
Endocytosis
Process of engulfing substances into the cell.
Primary lysosomes (heterophagy)
Lysosomes before fusion with late endosomes.
Secondary lysosomes (heterophagy)
Lysosomes after fusion with late endosomes.
Virchow
Historian who studied white blood cells
Examined pus and saw that WBC with RBC inside
said that white blood cells were giving birth to red blood cells
Metchnikoff
Father of probiotics
said that WBC were not giving birth
WBC are actually undergoing phagocytosis
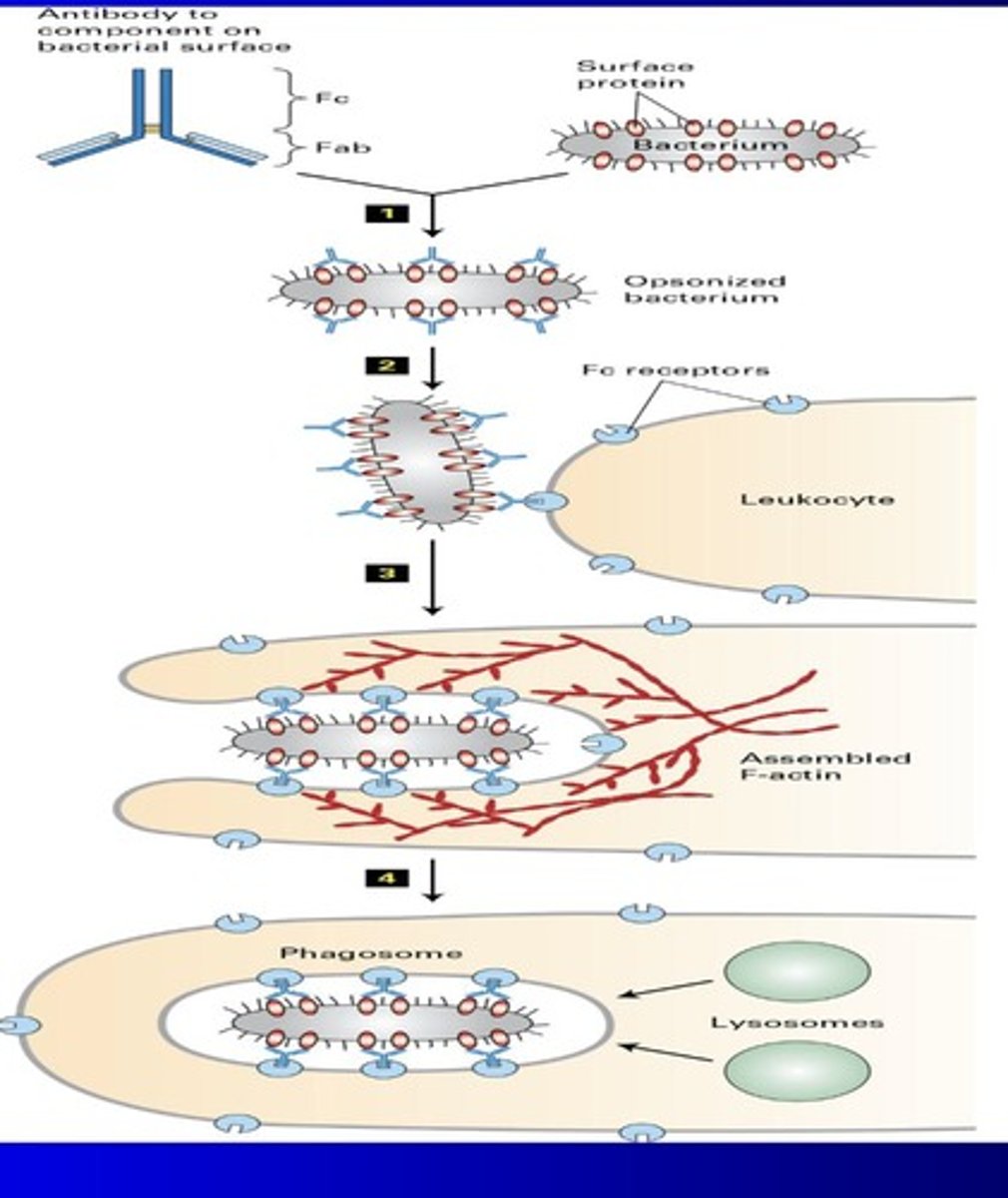
Opsonization (metchnikoff)
Coating bacteria with antibodies to enhance phagocytosis.
Abs bind to FC receptors and WBC
WBC undergoes endocytosis of bacteria
lysosomes in WBC destroy bacteria
Myasthenia Gravis
autoimmune disorder where immune system attacks muscular junction/neuromuscular transmission
Causes of myasthenia Gravis
Many causes but mainly due to ACh being targeted by Abs and can’t bind to AChR or because AChR is damaged
AChR
Acetylcholine receptor targeted in Myasthenia Gravis.
Droopy eyelids
Common symptom of Myasthenia Gravis.
Experiment for myasthenia gravis
Curare: Plant derivative that blocks acetylcholine receptors.
People with MG more sensitive to this derivative than healthy people (i) When ACh can’t bind to receptor, muscle contraction and movement is inhibited (ii) This is already the case for people with MG so curare makes it worse (b) Acetylcholinesterase (AChE)inhibitors (example: eserine) increases ACh availability, allowing for relief for patients with MG
AChE inhibitors
Drugs that increase acetylcholine availability.
Miniature end plate potential (MEPP)
Measurement of ACh in neurotransmitter vesicles.
2mV for heathy ppl
1mV for MG pts
3H alpha bungarotoxin (experiment for MG)
Radioactive substance used for ultrastructural autoradiography
Scientists labeled α-bungarotoxin with radioactive isotopes or fluorescent dyes.
They used it to visualize AChRs at neuromuscular junctions under microscopes.
showed less AChR in MG patients than healthy ppl
Electroplax (experiment for MG)
Part of electric stingray with high AChR concentration.
Researchers used electroplax tissue from electric fish to extract large amounts of pure acetylcholine receptors.
AChR purified from electroplax and added into mice that were injected with serum from MG patients (c) Mice showed MG symptoms showing that just adding AChR won’t reduce MG symptoms (i) Anti-AChR Abs will bind to the AChR and induce heterophagy causing lysosomes to degrade and prevent them from being used
what did all of the experiments for MG show us
These experiments showed that MG is associated with low functioning ACh and AChR levels
AChR
Acetylcholine receptor, crucial for neuromuscular transmission.
Anti-AChR Abs
Antibodies that bind AChR, impairing function.
Heterophagy***
Process where lysosomes degrade unwanted cellular components.
Neostigmine (treatment for MG)
Inhibits acetylcholinesterase (ACh esterase), increases Ach half life
In MG, the problem is that ACh can’t activate enough receptors (because antibodies have blocked, destroyed, or reduced them).
So if ACh is broken down too quickly by AChE, there’s even less chance it’ll find and activate the few functioning receptors that are left.
Prednisolone (treatment for MG)
Corticosteroid that decreases immune response
Vyvgart (treatment for MG)
Blocks IgG binding to ACh, preventing degradation***
Capillary endothelial cells bind to IgG molecules (i) This prevents them from binding to ACh (a) ACh gets degraded when IgG molecules bind to them
Autophagy
Cellular process for degrading and recycling organelles.
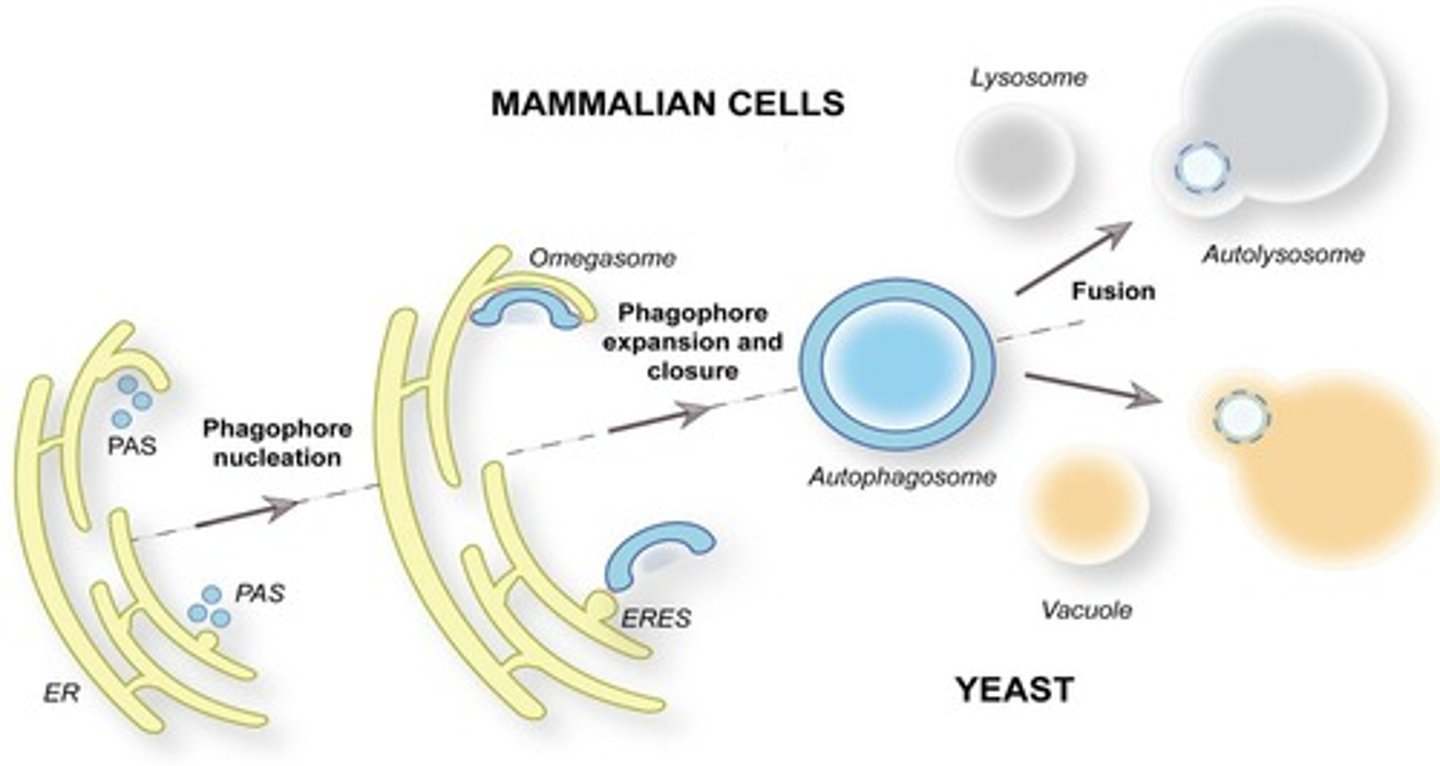
Yoshinori Osumi
Scientist who discovered autophagosomes (a double-membrane vesicle formed during autophagy, a cellular process where the cell breaks down and recycles damaged or unnecessary components. It engulfs cytoplasmic material and delivers it to lysosomes for degradation.
Responsible for organelle turnover a) When organelles die autophagy destroys it b) M.T half life 5-6 days c) Pathway
Phagophore
Membrane structure initiating the autophagy process.
(a) Membrane bound
(b) Starts autophagy
(c) Comes from RER
emerges from the RER and becomes autophagosome
LC3 proteins
Regulate formation of autophagosomes.
many druges will mess with autophagosomes
Chloroquine
Drug affecting autophagy
Can make cancer cells more sensitive to cancer drugs but can also harm normal cells
Myopathy (side effect of chloroquine)
Muscle disease causing decreased strength.
Retinopathy (side effect of chloroquine)
Damage to the retina, affecting vision.
Silicosis (lysomal disease, nongenetic/environmental)
Also known as Miner’s disease
Lung disease caused by silica being inhaled in the coal mines
causes fibrosis
Fibrosis
Thickening and scarring of connective tissue.
(i) Increased collagen being released in the lungs (ii) Lungs become less elastic and decrease CO2 O2 exchange
experiment done for silicosis/fibrosis
(i) Cells placed in culture with silica which is naturally sharp (ii) Cells will endocytose the silica and the lysosomes used to transport them will puncture and become leaky and the cell dies due to hydrolytic enzymes spilling out (a) Can be resolved via collagen
Inherited lysomal storage diseases
about 70 different types
most common treatment is enzyme replacement therapy (ERT)
Tay Sachs (inherited lysomal disease)
Lysosomal storage disease due to Hex A deficiency.
Ganglioside GM2 builds up in lysosomes
symptoms of Tay Sachs
(a) Hypotonia
(b) Red spots in the retina
(c) Startled by loud noises
(d) Blindness
(e) Deafness
NO ERT YET BUT GENE THERAPY HAS BEEN SUCCESSFUL
Hurler syndrome (inherited lysomal disease)
Genetic disorder caused by alpha-L iduronidase deficiency.
Those with this syndrome look like gargoyles
Aldurazyme is enzyme replacement therapy for hurler syndrome
Experiment for Hurler syndrome
(a) Hurler cells placed on top of culture (b) Normal cells on bottom (c) Over time hurler cells started to look like normal cells (d) This experiment was the basis for ERT
I cell disease (inherited lysomal disease)
Very rare
caused by failure to add M6P
M6P is a tag that gets added to certain enzymes in the Golgi apparatus.
This tag is like a shipping label — it tells the cell to send those enzymes to the lysosome, where they’re needed to break down various substances
Symptoms of I cell disease
same as Hurler but more severe
skeletal abnormalities
I cell disease experiment (figure out)
(a) If someone knows what the experiment was that would be great (b) What was observed (i) I cells have lysosomes without hydrolytic enzymes (ii) (iii) Excess lysosomal enzymes were found outside the cell I cells can endocytose lysosomal enzymes (a) Fibroblasts can’t
What we know from I cell disease experiment
Because of all of this we know that the N-acetyl glucosamine pathway in the cis golgi is what is affected by this disease
Gaucher disease (inherited lysomal disease)
Condition caused by glucocerebrosidase deficiency
Cerezyme is the ERT
Cerezyme
Enzyme replacement therapy for Gaucher disease.
Pompe disease (inherited lysomal disease)
Disorder from alpha-glucosidase deficiency, affecting muscles
(a) Normally converts glycogen to glucose (b) Without it, there is really only glycogen (c) Glycogen stays in lysosomes until converted to glucose (d) Muscle cells require lots of glucose (2) Causes giant lysosomes in muscle cells
Proteasomes
Degradative organelles
1. Degrades ubiquitinated protein (UPS pathway) 2. Over 30k types 3. 750,000 daltons 4. Experimentation showed that yeast cells without proteasomes died
Velcade (experiment with proteasomes
Proteasome inhibitor used to treat cancer
Velcade inhibits the 26S proteasome, preventing protein breakdown.
This causes a toxic buildup of proteins inside the cell.
Cancer cells, especially myeloma cells, are very sensitive to this stress and undergo apoptosis (programmed cell death).
Localized energy control
Efficient management of energy production in cells.
Mitochondria
1. can appear as many shapes
-Co sediments with lysosomes
2. Not uniformly distributed in the cell
- Cell 1 can have MT on the right side and cell 2 can have it on the left
3. MT attached to microtubules
4. MT DNA has 13-37 genes
5. MT consists of nuclear encoded proteins
HEME (protein inside MT)
(1) HEME synthesized by MT DNA, shipped out the MT, modified outside the MT, brought back in the MT b) Why haven’t MT lost their genes? (1) Efficient localized control of energy production (2) Evolution favored these genes
Heme plays a crucial role in the mitochondria by enabling electron transport and energy production. It is a key component of cytochromes in the electron transport chain, where it helps transfer electrons and drive ATP synthesis. Heme also binds and reduces oxygen at Complex IV, allowing cells to safely use oxygen without generating harmful reactive oxygen species
Mitochondrial structure
Includes outer and inner membranes with distinct properties
Outer membrane
Contains few proteins and is highly porous.
Porin
Allows molecules under 5,000 daltons to enter the outer membrane
Inner membrane
Rich in proteins, crucial for energy processes
ETC
5x the surface area compared to outer membrane
Cardiolipin
A four-legged fatty acid
Increases electrical resistance of the membrane
Fission
a) A way to distribute MT to daughter cells during mitosis
b) A way damaged parts of MT can be removed
Fusion
a) Maintains a homogenous pool of MT in the cell
b) Could restore function of damaged MT
c) Plays a role in where MT is located in the cell
ER mitochondria associated membranes (MAM)
Regulates mitochondrial fission and lipid metabolism
a) Deals with
(1) MT fission
(2) Ca2+ homeostasis
(3) Regulates lipid metabolism, autophagy and mitophagy
(4) Alzheimers and parkinsons
Nanotubes
Facilitate mitochondrial transport between two different cells.
Mitochondrial permeability transition pore (MPTP)
Opens under stress (extrinsic), triggering apoptosis via cytochrome C.
(1) Note: this is extrinsic cell death because it is caused by an external stressor/factor
(2) MPTP is closed and cytochrome C is located inside the MT
(3) Stress causes MPTP to open and cytochrome C goes into cell cytoplasm and binds to APAF1
(4) APAF1 binds to procaspase 9 and activates it to become caspase 9
(5) Caspase 9 now activates procaspase 3
(6) Procaspase 3 gets activated and turns to caspase 3 which is known as an executor enzyme
(7) Cell undergoes apoptosis
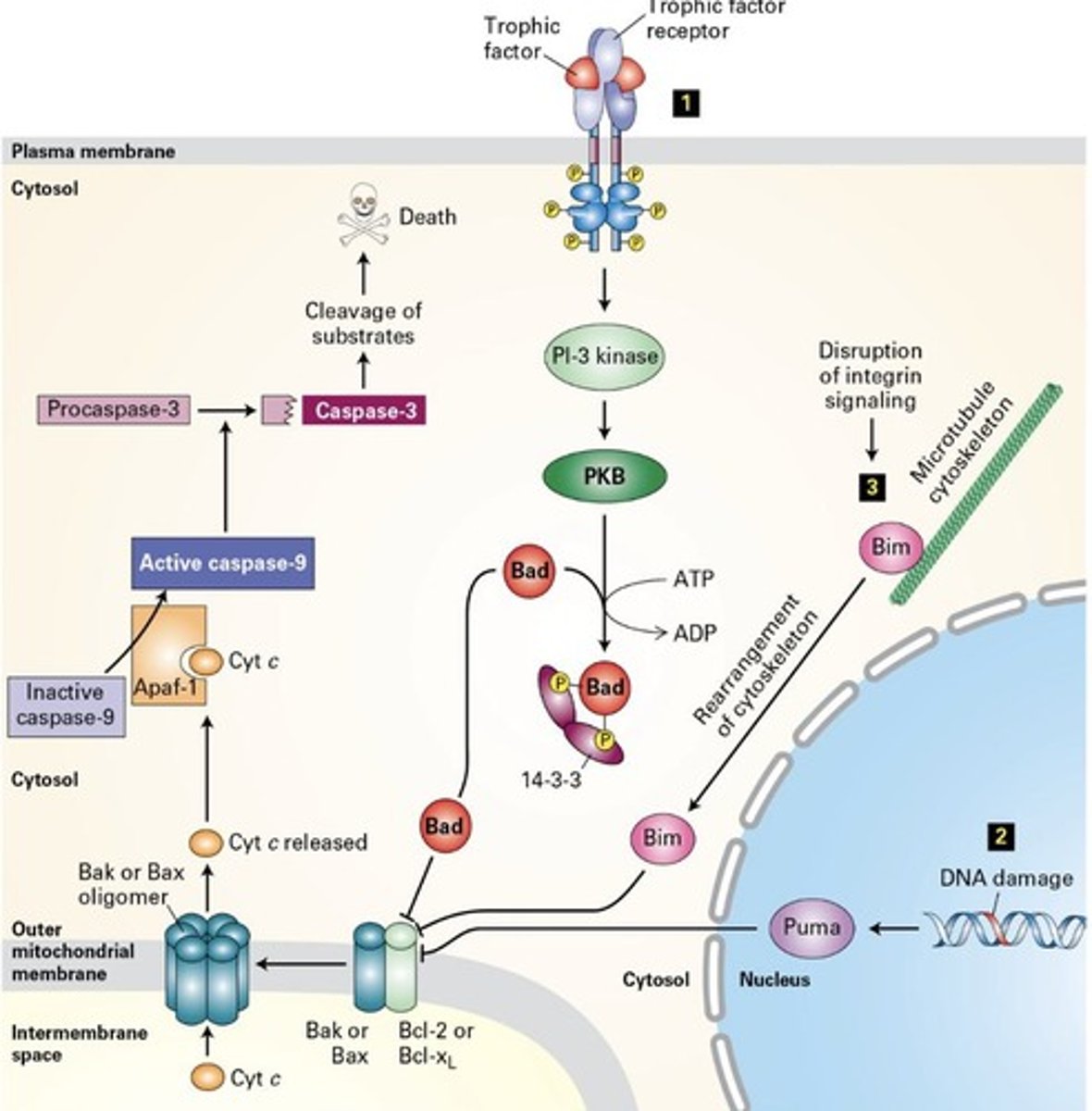
Cytochrome C
Releases from mitochondria to activate apoptosis pathway.
Caspase 9
Activates procaspase 3, leading to cell death.
MAIN TRIGGER
Bcl2
Anti-apoptotic protein linked to cancer development.
First discovered in B cell lymphoma and is being looked at as a target drug for cancers
cyclosporin A
inhibits MPTP
targets MPTP
used to transport STEM cells
Genasense
Bcl2 antisense used to prevent Bcl2 production by targeting the mRNA
Many cancers (like melanoma, leukemia) overexpress BCL-2 to avoid dying
Genasense binds BCL-2 mRNA and blocks translation, reducing BCL-2 protein levels
This should promote apoptosis in tumor cells
Obatoclax
Inhibitor of Bcl2, used in cancer therapy.
Venclexta
Treats chronic lymphocytic leukemia by targeting Bcl2.
Elesclomol
(1) Increases oxidative stress in MT by targeting ETC and causing apoptosis
2. Most types of cancer overexpress Bcl2
3. Bcl2 is an anti-apoptotic protein
Warburg effect
1. Cells use glycolysis over the MT even when there is oxygen
2. Glycolysis is optimized to get the most ATP a) More than cellular respiration
3. This is one of the reasons why cancer cells live a long time
4. Warburg effect is why HEPG2 cell line behaves differently a) Overexpression of GLUT 1 and 3 b) Overexpression of LDH c) Less processes in MT matrix
Mitochondrial diseases
1. Around 250 of them
2. Inherited solely through mother
3. mtDNA does not use correction enzymes
4. Large link between mtDNA mutations and autism
5. MT dysfunction linked with alzheimers
- a) Defect in TCA allows for beta amyloid build up which causes alzheimers
Glycolysis
Produces 2 ATP and NADH from glucose breakdown
NADH from cytoplasm gets converted to ATP via 2 antiporters
ETC inhibitors (drug that targets the MT)
Substances like cyanide that block electron transport.
CO
Proton uncouplers
Compromise H+ gradient, affecting ATP synthesis.
Proton uncouplers are molecules that disrupt the link between the electron transport chain (ETC) and ATP production in mitochondria.
DNP (proton uncoupler)
(1) Lipid soluble
(2) Weak acid
(3) Shuttles H+
(4) Proton ionophore
(5) Used to be used for weight loss
(a) OG ozempic
(b) People burned up and died when taking it
UCP-1 (proton uncoupler)
(a) Natural analog of DNP
(b) Compromises H+ gradient
(c) Thermogenin
(i) Drug that uses UCP-1
(ii) Helps warm up shivering babies
valinomycin/gramicidin (protein uncoupler)
(1) K+ ionophore
(a) Compromises MT transmembrane voltage of 200mV
Oligomycin (protein uncoupler)
Blocks flow of H+ in oxidative phosphorylation
PS inhibitors (protein uncoupler)
(1) Cycloheximide
(a) Blocks nuclear translation
(2) Tetracycline
(a) Blocks MT translation
Cellular respiration
Process converting glucose into ATP via multiple stages.
cellular respiration process
a) Glycolysis
(1) Happens in cytoplasm
(2) Glucose broken down into 2 pyruvate using ATP
(3) Net gain of 2 ATP and 2 NADH
pyruvate oxidation
(1) Pyruvate goes into MT via antiporters
(2) Will be decarboxylated to form CO2 as byproduct
(3) Will be dehydrogenated by NAD+ to form NADH
(4) CoA will bind to form Acetyl-CoA
Pyruvate oxidation turns pyruvate into Acetyl-CoA, releases CO₂, and makes NADH — it's the link between glycolysis and the Krebs cycle.
Krebs cycle
Converts Acetyl-CoA into 2 CO2, 3 NADH, 1 FADH, and 1 GTP or ATP.
The Krebs cycle (also called the citric acid cycle or TCA cycle) is the part of cellular respiration that produces energy by breaking down Acetyl-CoA (from food like glucose or fatty acids) and releasing carbon dioxide.
happens in mitochondira
Inside out mitochondria particles
1. We can’t use MT for experiments so we turn them inside out for experimentation
2. How to make them a) Isolate MT
b) Place in low osmolarity solution
c) MT will flip inside out
(1) MT will have F0 and F1 proteins at this point on the inside out membrane
use pH sensitive dyes
ETC
Electron Transport Chain; crucial for ATP production.
(1) FADH and NADH release electrons through protein chain
(2) Causes shift in proton gradient
(3) H+ will go through transporters that will phosphorylate ADP into ATP
(a) ATP synthase specifically
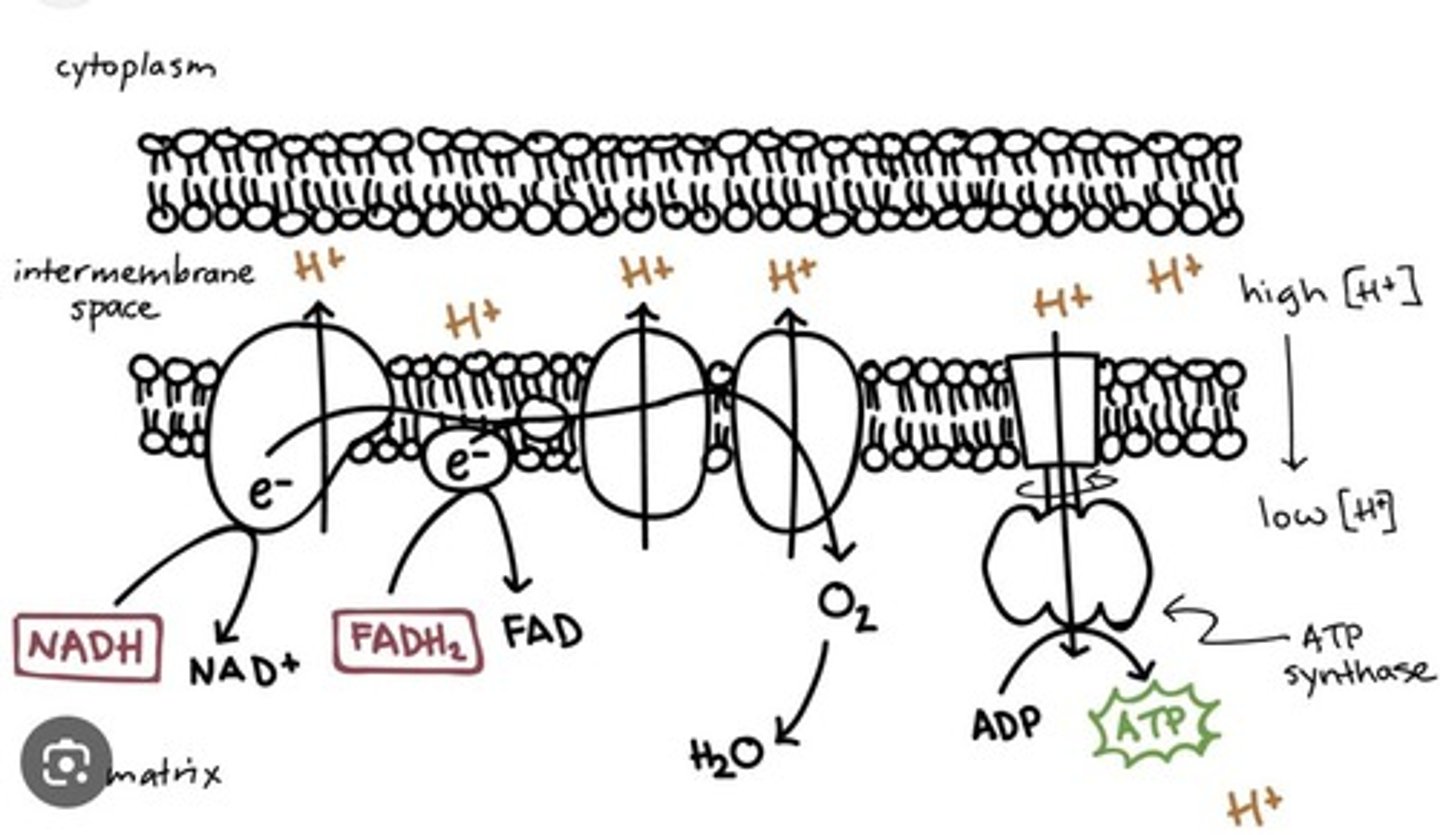
ADP phosphorylation
Conversion of ADP to ATP via phosphate addition.
Radioactive PO4
Phosphate used to trace phosphorylation processes.
MT functionality
Mitochondrial function assessed by ATP production efficiency.
Validamycin
Inhibits voltage and ATP production in cells.
DNP
Disrupts voltage and H+ gradient, reducing ATP.
Vm
Membrane potential, additive with H+ gradient.
Mechanical agitation
Separates F0 and F1 ATP synthase components.
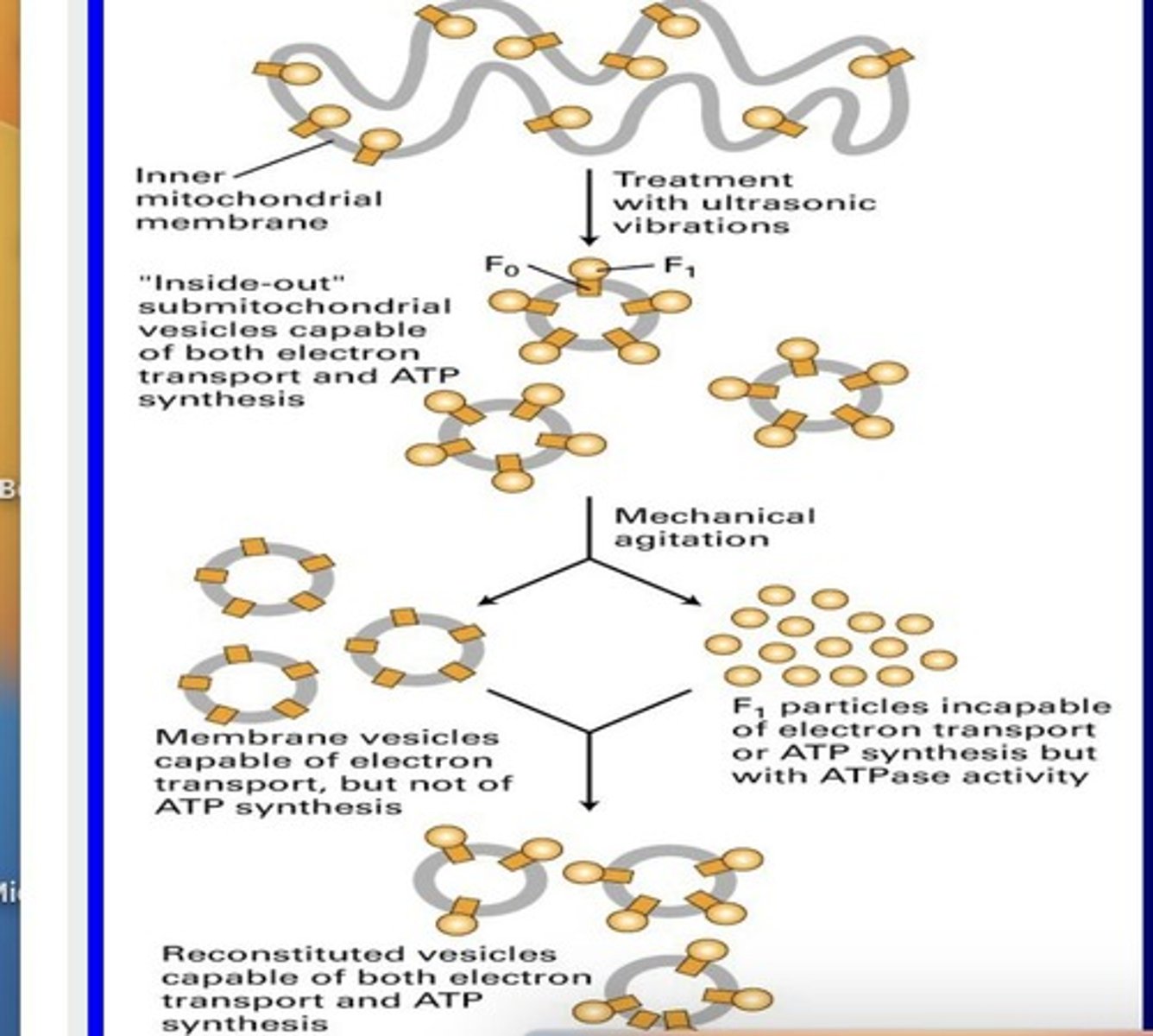
F0 particles
Have working ETC but no ATP synthesis.