Periodic Table (The Periodic Table)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
As you go down group 1
Softness increases, density increases, melting point decreases, reactivity increases.
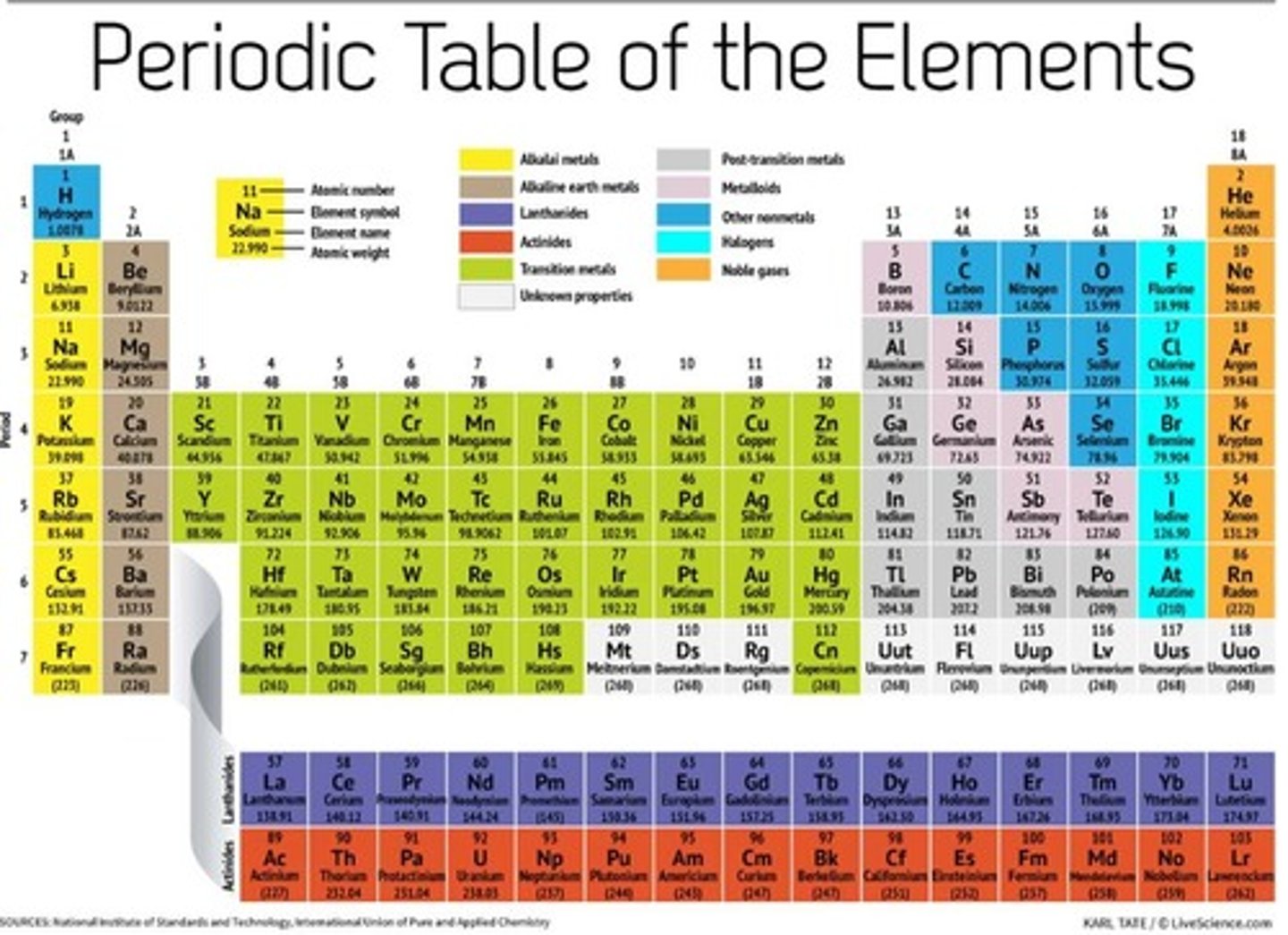
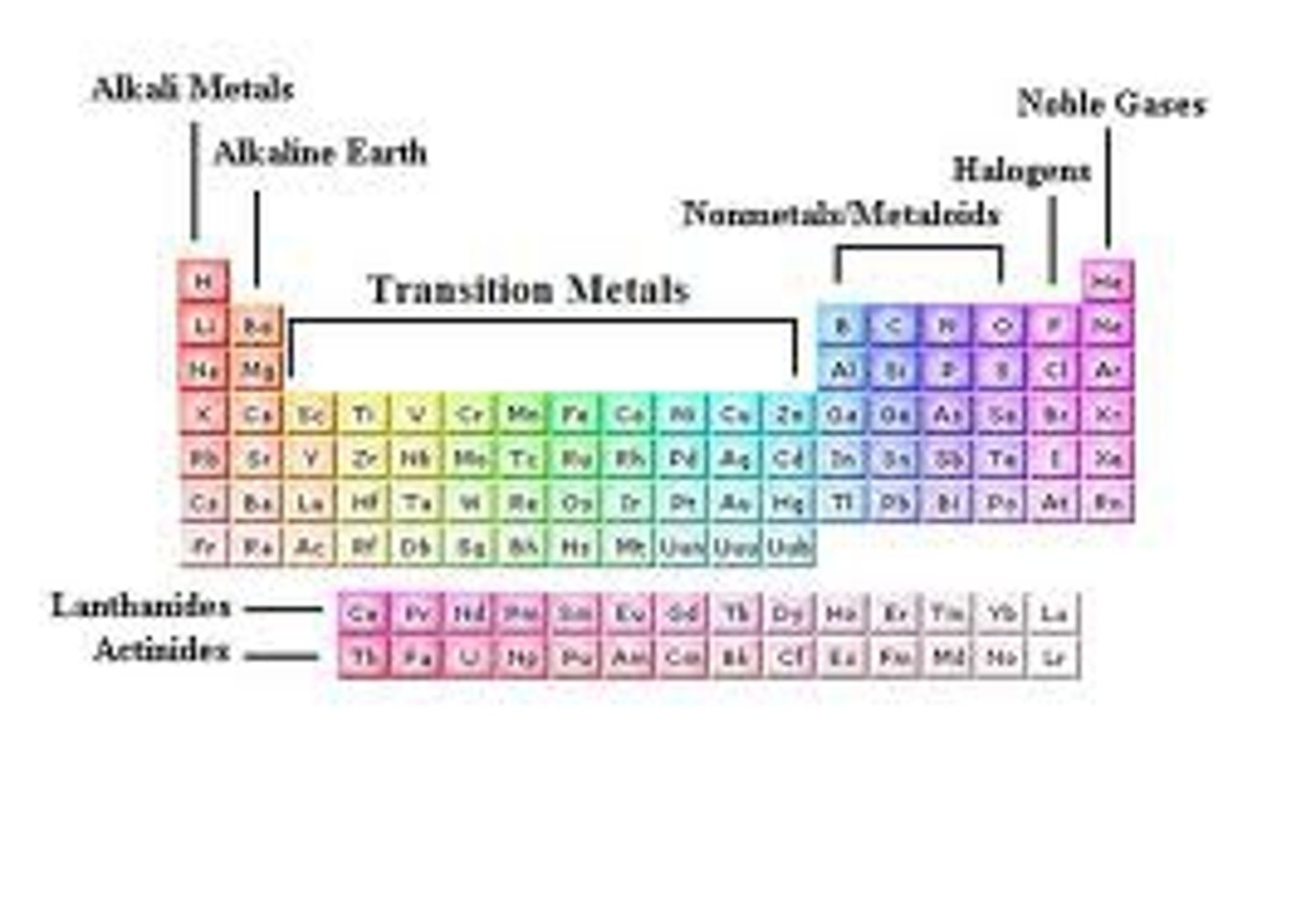
Group 1
Alkali metals.
Atoms with in the same group
React in the same way
Group 17
The halogens
Group 18
Noble gases
Group 2
Alkaline earth metals
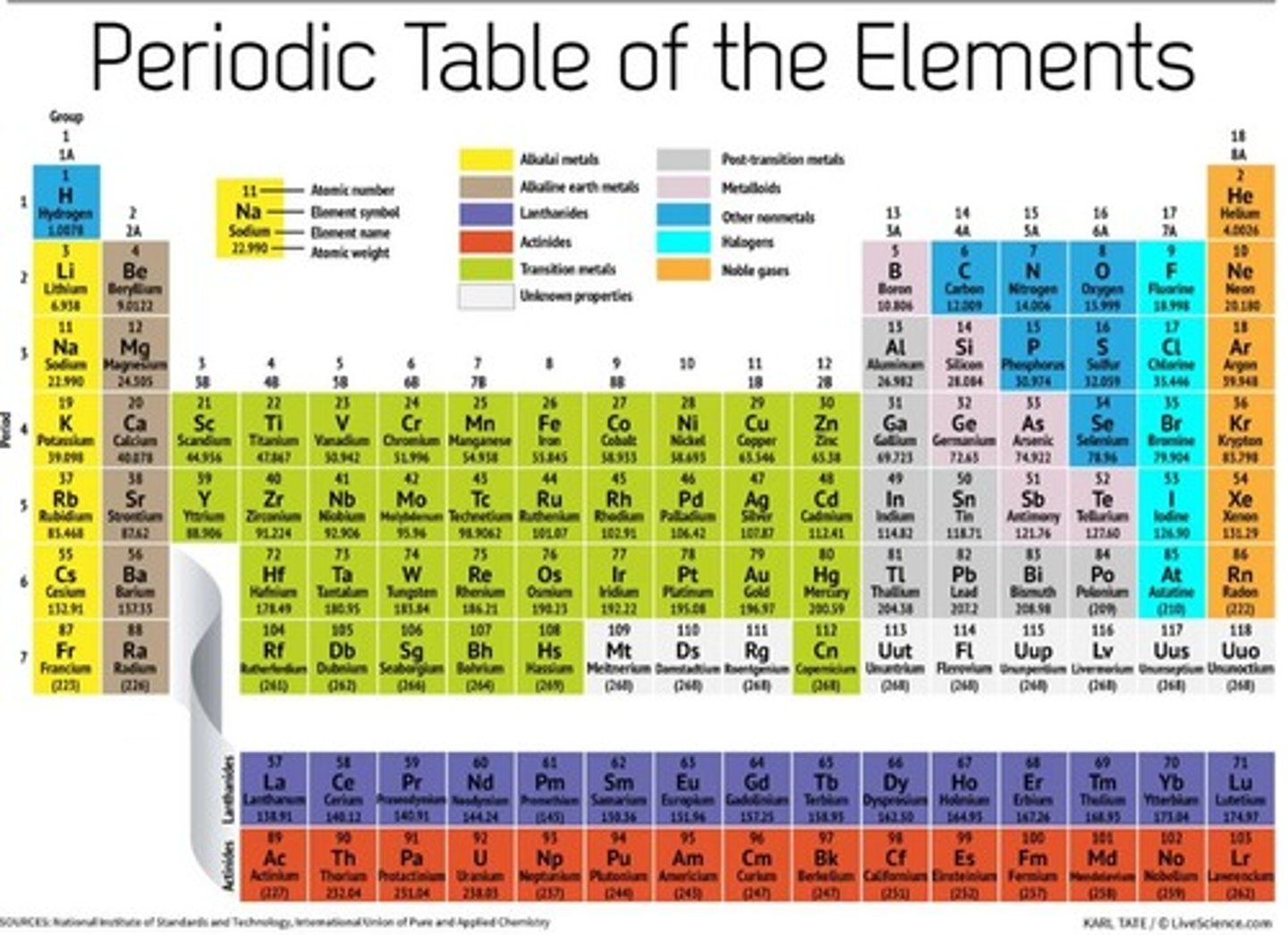
Groups 3-12
transition metals
metalloids
Found along the 'staircase'. Have properties of both metals and nonmetals
nonmetals
brittle , dull, poor conductors of heat and electricity, and have much lower melting and boiling points
Proton
Positively charged particle in the nucleus of an atom
Neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
Electron
A tiny, negatively charged particle that moves around the nucleus of an atom.
Atomic Number
The number of protons in the nucleus of an atom of an element

Mass Number
The total number of protons and neutrons in an atom's nucleus

Periodic table
A chart of all chemical elements currently known, organized by their properties.
Element
pure substance that consists entirely of one type of atom
atom
the smallest unit of an element that maintains the properties of that element
group
oxygen and sulfur are in the same ______
periods
horizontal rows on the periodic table are called ____________
groups
vertical columns on the periodic table are called __________