Chem 1 test (103)
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
Matter
anything that has mass and occupies space
Chemistry
the study of matter
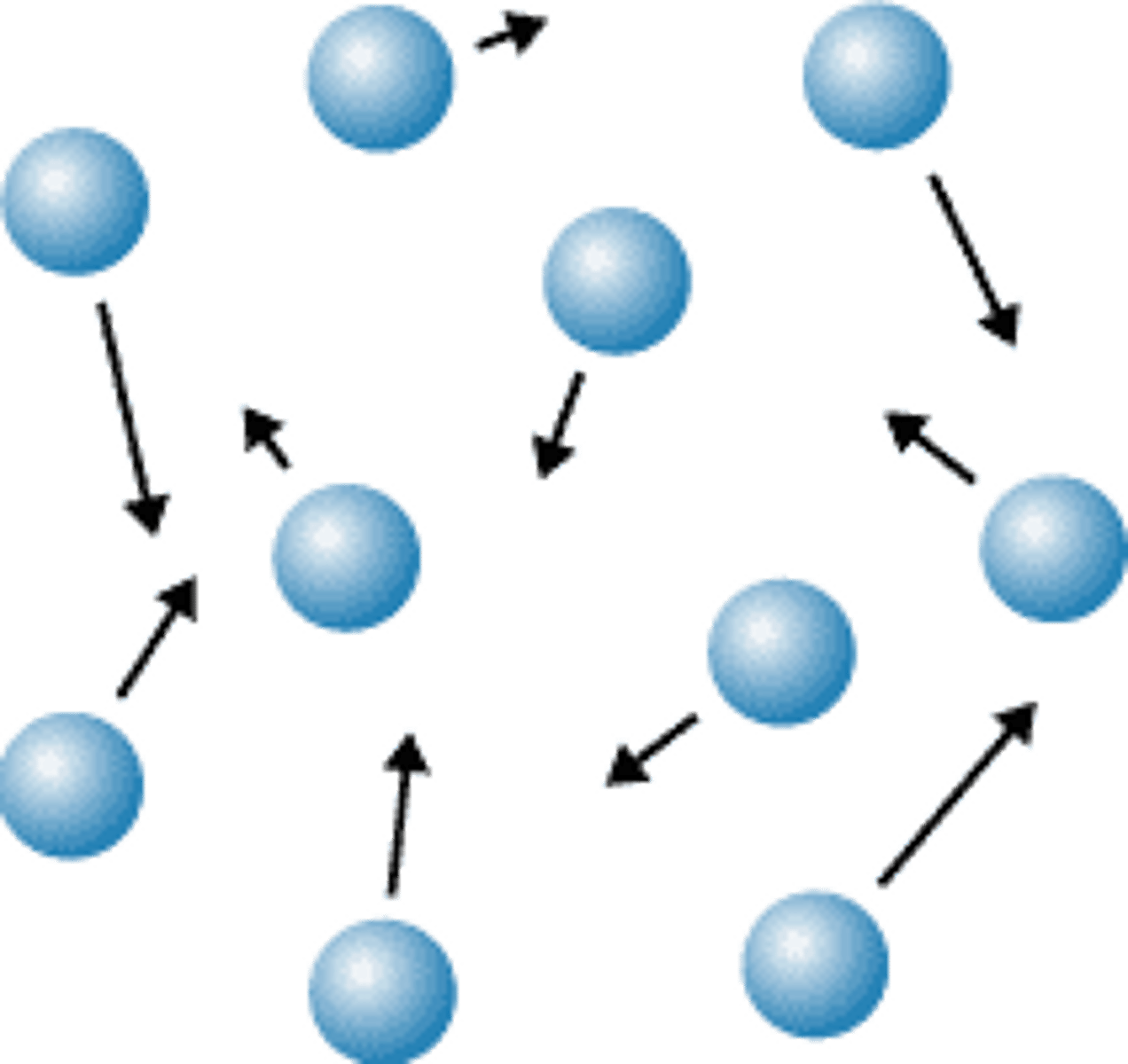
solid

liquid
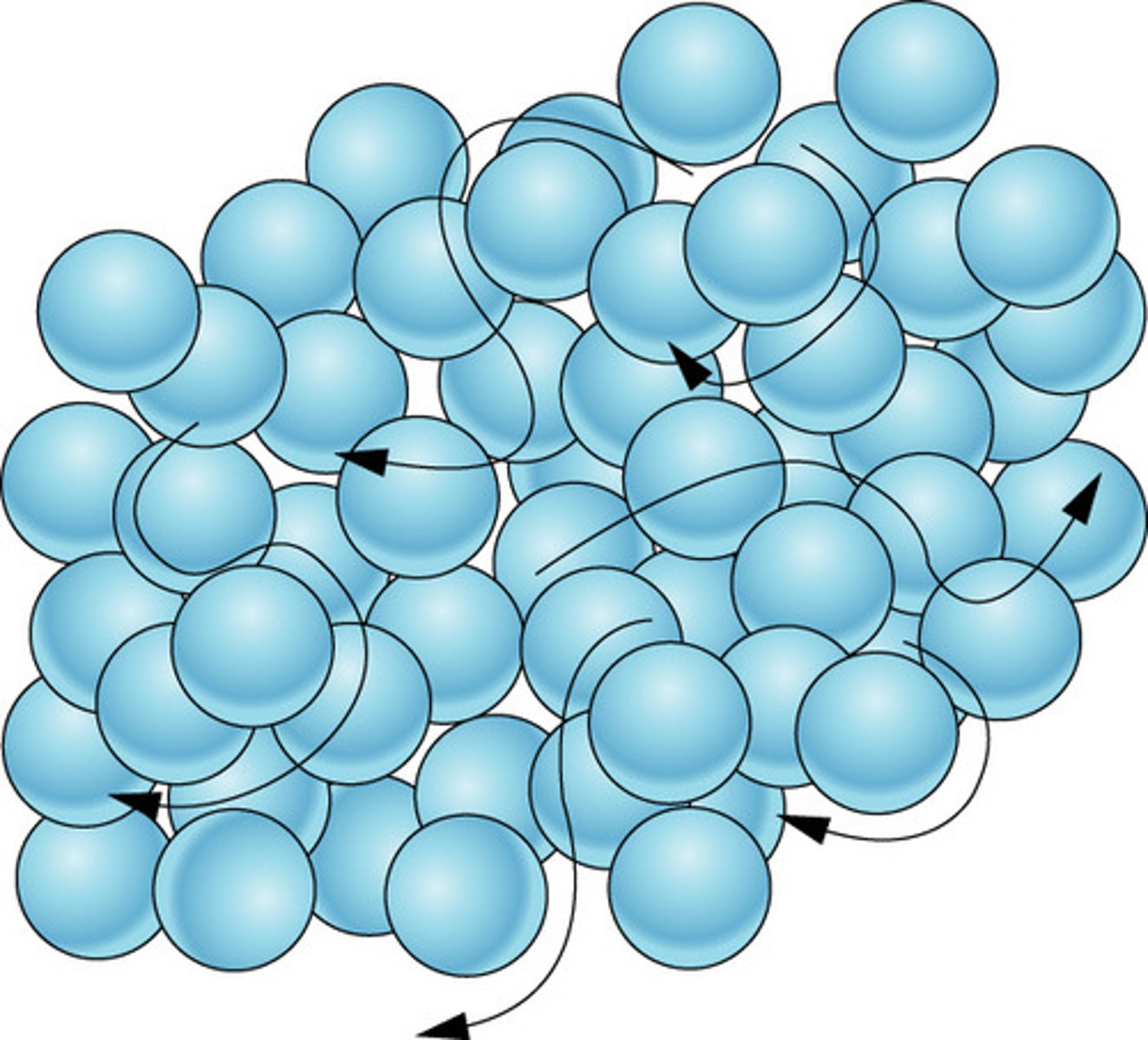
gas
physical properties
can be observed or measured without trying to change the composition of the matter being studied (color, size, volume)
chemical properties
Demonstrated when attempts are made to change matter into other kinds of matter (acidity, reactivity, flammability)
Physical changes
change matter undergoes without changing composition
Chemical changes
Change matter undergoes that involves changes in composition
Pure substance
has constant composition and fixed properties (water, sugar, salt)
Mixture
A blend of matter that can be physically separated into its separate components (sugar water)
Homogenous matter
matter that has the same properties throughout the sample, they are solutions (uniform)
Heterogenous matter
matter with properties that are not the same though out the sample (non-uniform)
Examples of Homogenous matter
air, milk, soda, IV/saline solutions
Examples of Heterogenous matter
Humans, blood, steak
Elements (pure substance)
"building blocks" consisting of one type of atom in the form of homoatomic molecules or individual atoms (cannot be broken down further with chemical means)
Atoms
The limit of chemical subdivision for matter "building blocks of elements"
Compounds
A pure substance consisting of 2 or more elements with definite composition that can be broken down into simpler substances by chemical methods
molecules
smallest particle of a pure substance that has properties of that substance and is capable of stable independent existence
Diatomic molecules
contains 2 atoms
Homoatomic molecules
contain 1 KIND of atom
triatomic molecules
contains 3 atoms
polyatomic molecules
contains more than three atoms
Heteroatomic molecules
contain 2 or more KINDS of atoms
tera-
10^12
giga-
10^9
mega-
10^6
kilo-
10^3
deci-
10^-1
centi-
10^-2
milli-
10^-3
micro-
10^-6
nano-
10^-9
Scientific notation
Represents numbers consisting of a product between a nonexponential number and 10 raised to a whole number exponent (m x 10^n)
sig figs
The number in a measurement that represents the certainty of the measurement, +1 number representing an estimate
sig fig rule for mult/div
Always use smallest number of sig figs presented in the problem for answer
sig figs for add/sub
use the smallest number of DECIMAL PLACES presented in the problem
Sig fig rules for zeros
leading zeros are non-significant but buried zeros are significant, trailing zeros require a decimal place to be significant
formula for Celsius to Fahrenheit
(9/5)(C)+32 =F
Percentage equation
part/whole x 100
part equation
(percent)(total)/100
Mixed calculation sig fig rule
According to PEMDAS, the last calculation determines the sig fig rule
Density equation
mass/volume
definition of density
physical property of matter
periods
horizontal rows on periodic table (similar electron config)
groups
vertical columns on periodic table (similar characteristics)
Characteristics of metals
high thermal/electrical conductivity, luster, ductile, malleable
Characteristics of non-metals
dull, brittle, poor conductivity
characteristics of metalloids
properties between metals/non-metals
Group 1A
Alkali metals
Group 2A
Alkaline earth metals
Group 7A
Halogens
Group 8A
Nobel gases
Coulomb's Law
Opposites attract, likes repell
Atomic number
shows number of protons
Atomic weight
The mass of the weighted average of the atoms (naturally occurring isotopes) of an element
Isotopes
Atoms that have the same atomic number but different mass numbers (different number of neutrons)
finding atomic weight:
(% isotope)(atomic mass) + (% isotope)(atomic mass)
Radioactive nuclei
nuclei that undergo spontaneous changes and emit energy as radiation (unstable)
Radioisotopes
An isotope of an element that emits nuclear radiation
Radioactive decay
Process in which an unstable nucleus changes energy states and emits radiation in the process
Alpha particles (+)
Identical to helium nucleus and composed of 2 protons and 2 neutrons (most massive, highest charge, don't penetrate paper/skin cells)
Beta particles (-)
Identical to electrons but is produced in the nucleus when a neutron is charged into a proton and an electron (less collisions=more penetrating)
Gamma rays (no charge)
high-energy ray that is like an X ray but with higher energy. Not streams of particles but rays if electromagnetic radiation, very penetrating/dangerous
Nuclear reaction
When 2 atomic nuclei/ an atomic nucleus + another subatomic particle collide, resulting in +1 nuclei
positron
A positively charged electron, antimatter of an electron, penetrates similarly to beta particles (when a nucleus emits a positron, a proton is changed to a neutron, so the daughter nucleus has an atomic number lower by 1)
electron capture
A mode of decay for some unstable nuclei in which an electron from the outside of the nucleus is drawn in where it combines with a proton to make a neutron
Half-life
Time required for one half the unstable nuclei in a sample to undergo radioactive decay
Bohr Model
electrons are confined to clearly defined energy levels spaced at specific distances from the nucleus
atomic orbitals
volume of space around atomic nuclei in which electrons of the same energy may reside at any given time, groups with the same n value form subshells, each orbital can hold 2 electrons
valence electrons
the electrons in the highest period