Structure of the Atom
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
John Dalton
All matter made of tiny particles “atoms” which are indivisible and indestructible. Solid sphere
JJ Thomson
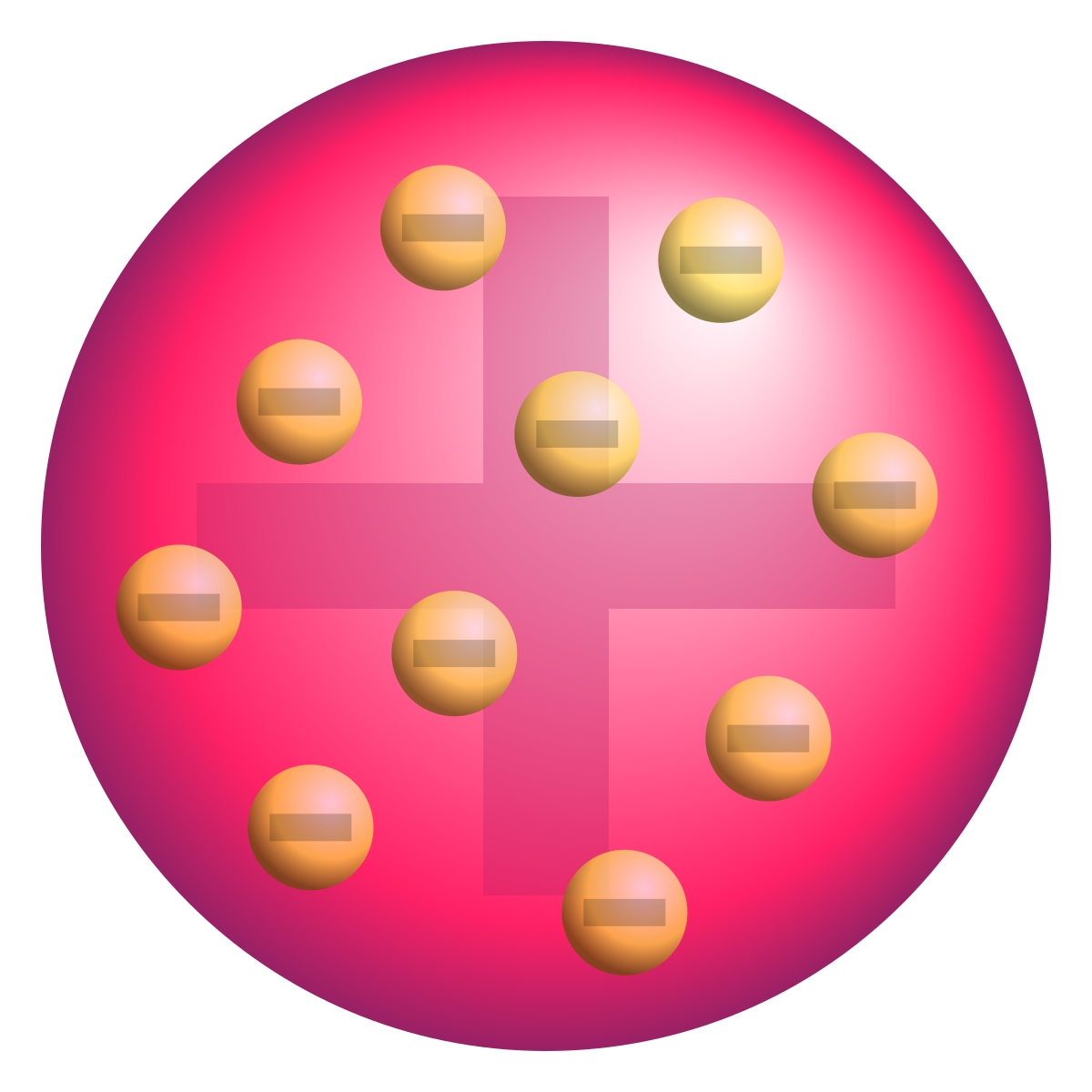
1904. Particles deflected by magnetic and electric fields which were small and negatively charged. Corpuscles - renamed electrons. Plum pudding model - atoms is a ball of positive charge with negatively charged ions within
Ernest Rutheford
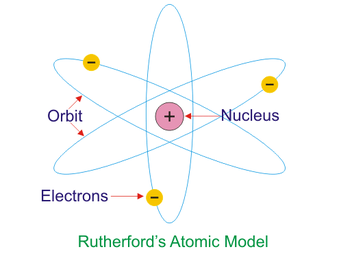
Gold foil experiment suggests that the atom has a small positive nucleus of protons with negative electrons moving around. atom was mostly empty. compact nucleus with negative charge surrounding.
Niels Bohr
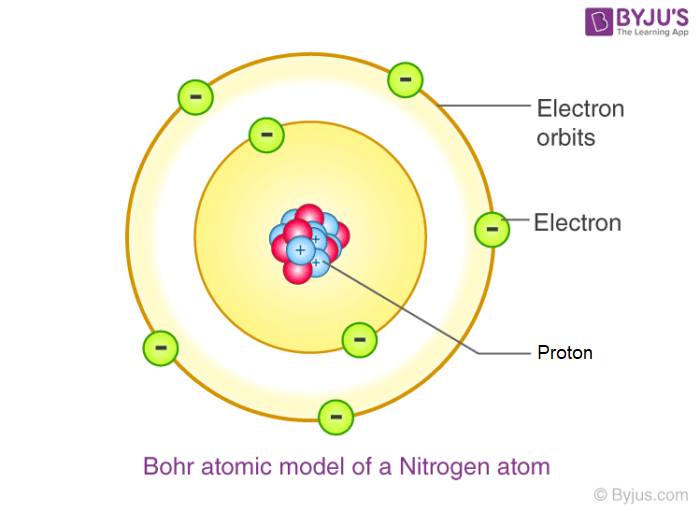
1913. Using Max Planck’s quantum theory to show that electrons orbit the nucleus. Chemistry of elements depend on number of electrons in outer most level.
James Chadwick
1932. Nucleus contains not 1 but 2 type of particles with identical mass. neutron - no charge. Protons and neutrons in the nucleus with electron orbits around.
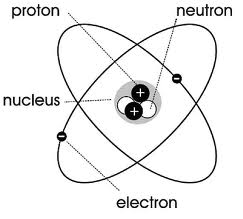
proton
Rutherford
Elecrton
Thomson
Neutron
chadwick
what is the trick to remeber the names and atoms
PEN = RTC