Electronic structure
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
What is electron configuration?
The arrangement of electrons in an atom.
Where are electrons arranged?
In principal energy levels, or principal quantum shells, numbered by the principal quantum number n.
How does n relate to energy and distance?
Lower n = closer to the nucleus, lower energy. Higher n = further away, higher energy.
Maximum number of electrons in shells?
n = 1 → 2
n = 2 → 8
n = 3 → 18
n = 4 → 32
What are subshells and how are they labelled?
Subdivisions of quantum shells, labelled s, p, d, (and f in heavy elements).
Order of subshell energy like which subshell gets filled first?
s < p < d < f.
What are orbitals?
Regions within subshells that hold up to 2 electrons with opposite spins.
Number of orbitals in each subshell?
s → 1 orbital (2 e⁻)
p → 3 orbitals (6 e⁻)
d → 5 orbitals (10 e⁻)
f → 7 orbitals (14 e⁻)
so multiply each number of orbtitals by 2 to workout total electrons in subshell
Shape of an s orbital?
Spherical; size increases with increasing shell number.
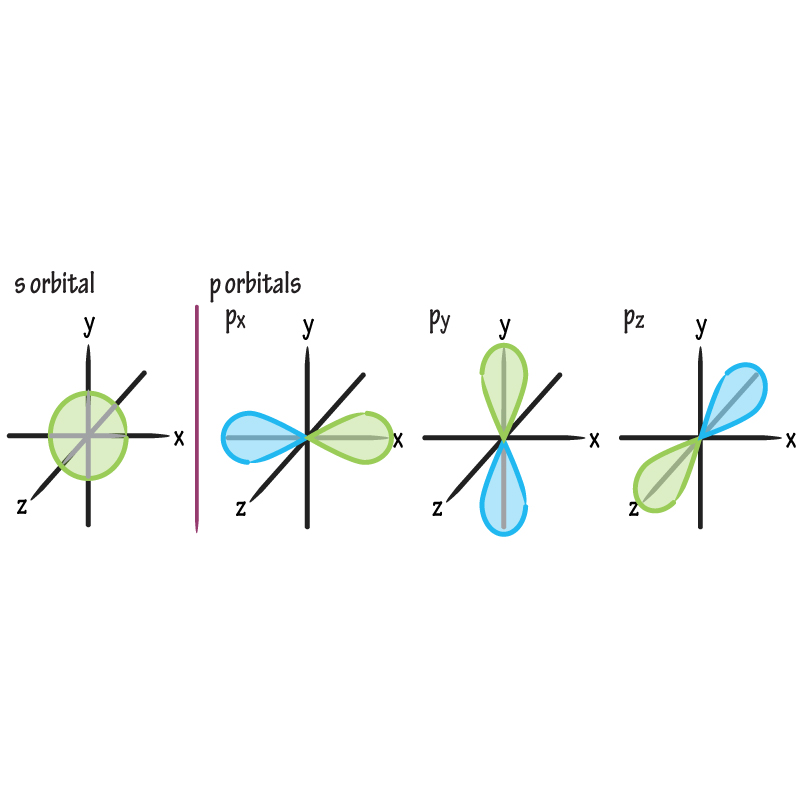
Shape of a p orbital?
Dumbbell-shaped; 3 per shell (either px, py, pz) at right angles; lobes increase with higher n. The px, py and pz orbital all make up the p subshell
What is electron spin?
Electrons rotate on their axis, creating a tiny magnetic field; can spin up or down.
What is Hund’s Rule?
Electrons occupy separate orbitals first with parallel spins before pairing up, so when two electrons occupy one orbital they spin in opposite directions
What is the Pauli Exclusion Principle?
An orbital holds max. 2 electrons, which must have opposite spins.
What does the principal quantum number tell us?
The energy level of a shell and energy of electrons in that shell.
Why do electrons pair in orbitals despite repulsion?
Because moving to a higher-energy orbital requires more energy than pairing.
What is the ground state?
The most stable electron configuration with the lowest energy.
Which orbital is filled first?
1s, then increasing order of energy levels.
What is an orbital spin diagram?
Diagram where each box = orbital; arrows represent electrons with opposite spins.
What are the two ways of writing electron configurations?
Full: Lists all subshells (e.g., 1s² 2s² 2p⁶).
Shorthand: Uses noble gas core in [ ] plus remaining configuration.
How are ions formed?
Negative ions (anions): gain electrons to outer subshell.
Positive ions (cations): lose electrons from outer subshell.
What’s unusual about transition metals’ electron loss?
General rule:
Normally, electrons fill orbitals in increasing energy order (Aufbau principle), e.g., 4s fills before 3d.
For most elements, this works perfectly.
Exceptions:
Some transition metals (like Cr and Cu) have electron configurations that don’t follow the expected order.
These are energetic preferences, not random — the system lowers its overall energy by promoting one 4s electron into the 3d subshell.
Chromium (Cr, Z=24)
Expected: [Ar] 3d⁴ 4s²
Actual: [Ar] 3d⁵ 4s¹
Reason: a half-filled 3d⁵ is more stable than 3d⁴.
Copper (Cu, Z=29)
Expected: [Ar] 3d⁹ 4s²
Actual: [Ar] 3d¹⁰ 4s¹
Reason: a fully filled 3d¹⁰ subshell is more stable than 3d⁹.
How is the Periodic Table divided by electron configuration?
s-block → valence electrons in s orbital
p-block → valence electrons in p orbital
d-block → valence electrons in d orbital
f-block → valence electrons in f orbital
Why do Cr and Cu have unusual electron configurations?
They adopt energetically favourable half-filled or fully filled d-subshells.
Actual configurations of Cr and Cu?
Cr: [Ar] 3d⁵ 4s¹ (not 3d⁴ 4s²)
Cu: [Ar] 3d¹⁰ 4s¹ (not 3d⁹ 4s²)
what is subshell notation?
diagram
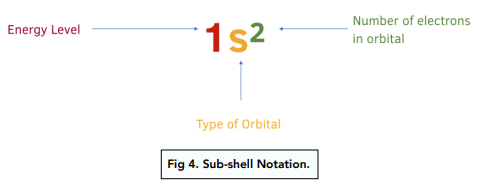
do we fill up the 4s subshell before the 3d subshell?
yes as the 4d is slightly lower in energy level
in an orbital spin diagram why do we fill orbitals singly first before pairing them up?
electron repulsion