aromatics 1&2
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
what are electrophiles?
"electron loving"
electrons flow towards electrophiles (they are electron acceptors)
They are a electron-deficient species
Usually positively charged
what are nucleophiles?
"nucleus loving"
electrons flow away from nucleophiles (they are electron donors)
They are a electron-rich species
Usually negatively charged
what is oxidation?
the removal of electrons from a molecule OR the addition of oxygen
what is reduction?
the gaining of electrons of a molecule OR the removal of oxygen
what are acids?
electron deficient species (positively charged)
what are bases?
electron rich species (negatively charged)
what is benzene?
Benzene is an aromatic compound
Its stabilised due to the delocalisation of π electrons (electron cloud)
Has equal energy resonance forms
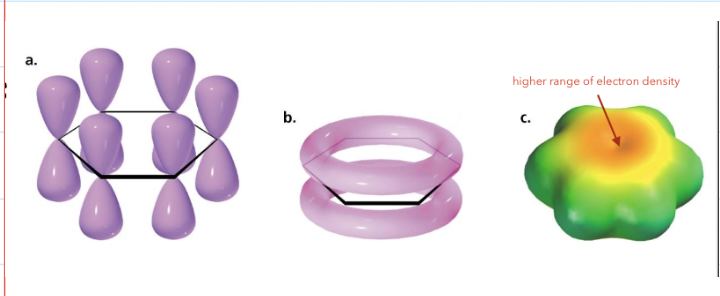
what are the general properties of aromatics?
Compounds have a uninterrupted cyclic cloud of p electrons above and below the plane of a planar molecule
The electron cloud contains an odd number of pairs of p-electrons
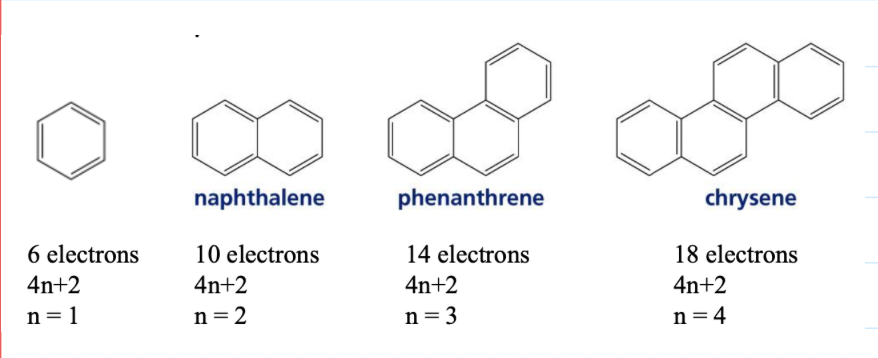
The p cloud will have 4n+2 electrons (Huckel's rule)
n = the number of rings
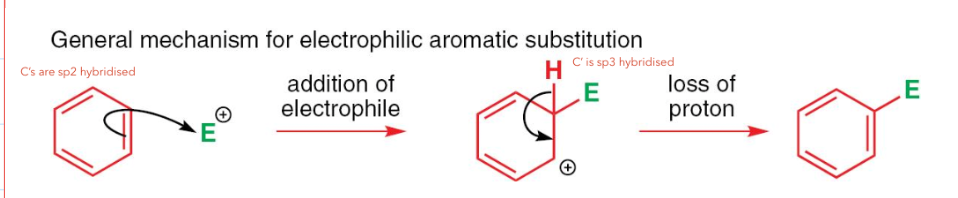
what type of reaction does benzene undergo and why?
electrophilic substitution
Benzene doesn’t undergo electrophilic addition like alkenes = this is due to the aromatic stabilisation
electrons are delocalised in the ring, so its hard to be susceptible to electrophilic attack
what conditions is needed for benzene to undergo electrophilic substitution and why?
This only occurs in the presence of an acid catalyst
The acid catalyst makes the electrophile more electrophilic
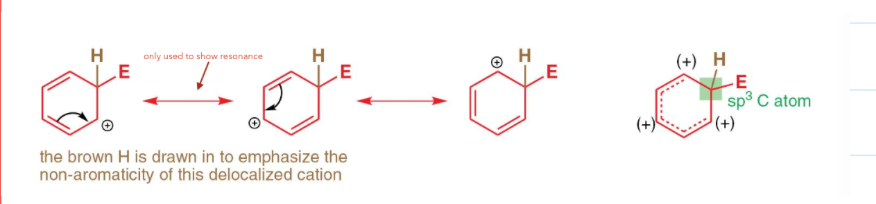
how is the wheland intermediate stabalised?
by resonance forms
draw the mechanism of electrophillic addition of an alkene
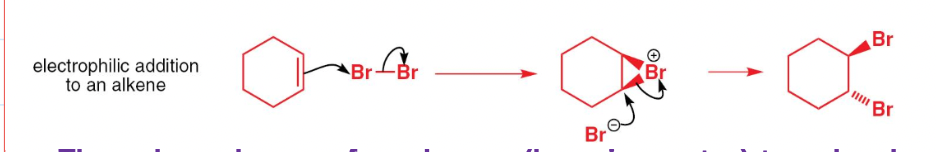
what colour change occurs when an alkene reacts with bromine water?
goes from organge-brown to colourless
draw the general mechanism of electrophilic substitution:
draw the resonance forms of benzene:
state the conditions needed for the nitration of benzene:
Concentrated HNO3
Concentrated H2SO4
draw the mechanism of the generation of the electrophile in nitration of benzene:
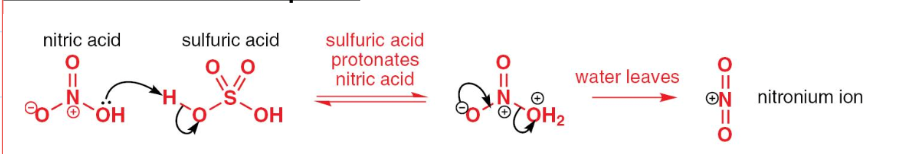
write the equation of the generation of the electrophile in nitration of benzene:
= HNO3 + H2SO4 ––> HSO4- + H2O + NO2+
draw the mechanism for nitration of benzene:
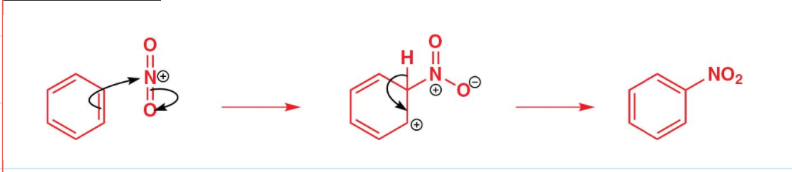
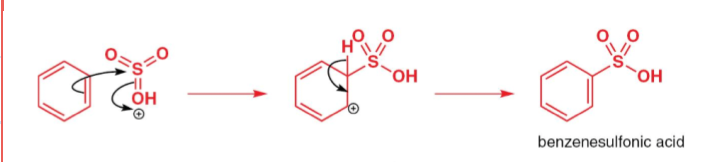
state the conditions needed for the sulfonylation of benzene:
Sulfur trioxide (SO3)
Concentrated Sulfuric acid (H2SO4)
draw the mechanism of the generation of the electrophile in sulfonylation of benzene:
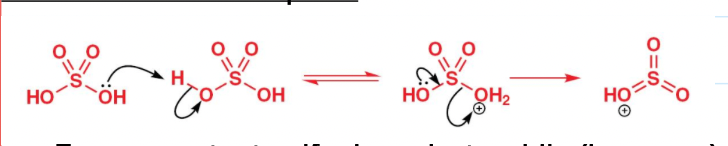
draw the mechanism for sulfonylation of benzene:
Deactivated benzene sulfonic acid is produced
what are the 2 types of Friedal-crafts reaction?
alkylation
acylation
what type of reaction does Friedel-Crafts undergo?
Ar-SE (electrophilic substitution reaction)
what does Friedal-crafts require?
a catalyst
preferably a Lewis acid
Draw the:
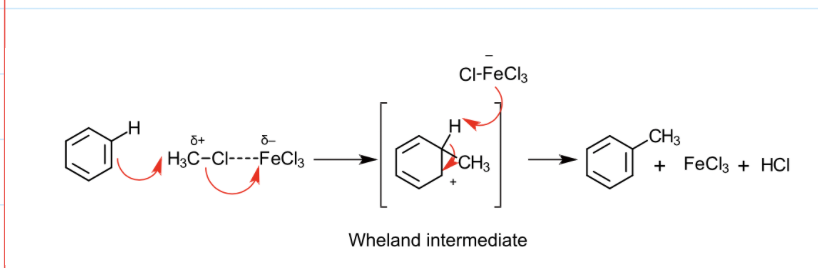
mechanism for the alkylation of alkyl halides (methyl chloride):
the generation of the electrophile
regeneration of catalyst
overll equation
CH3Cl + FeCl3 –––> CH3+ + FeCl4-
FeCl4- + H+ –––> FeCl3 + HCl
C6H6 + CH3Cl –––> C6H5CH3 + HCl
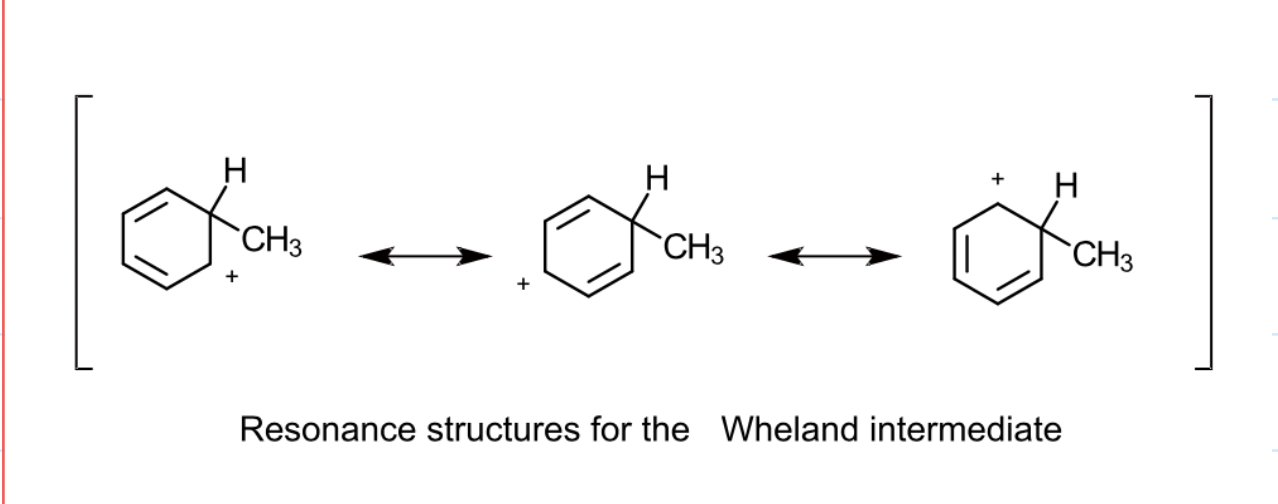
draw the resonance forms for the wheland intermediate in alkylation of methyl chloride:
draw the mechanism of action of the alkylation of n-propyl chloride:
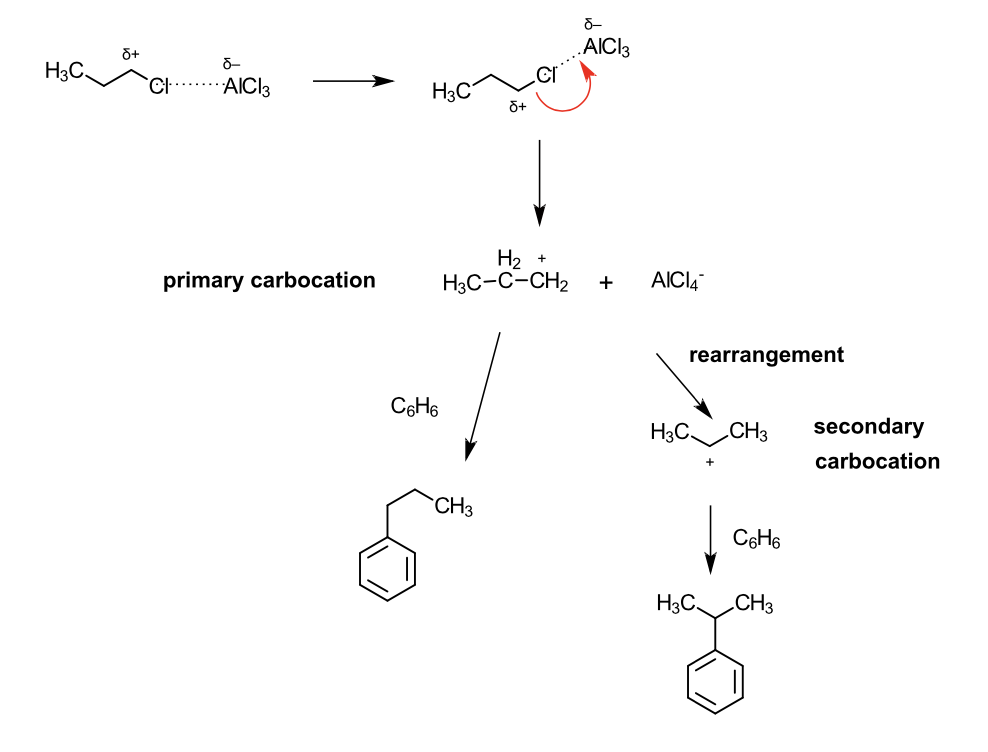
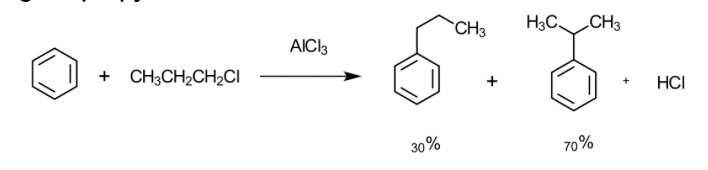
explain why this occurs:
Lewis acid (AlCl3) helps form a primary carbocation in the electrophile
Primary carbocation undergoes a shift to form a more stable carbocation (secondaryor tertiary)
The more stable it is, the more potent the attack is to the benzene ring
This forms a major product
It undergoes a SN1 reaction
what happens when monoalkylation takes place?
The resulting product is more reactive than benzene
Uses excess benzene - this increases the probability of an alkyl group reacting with an unsubstituted benzene
Possible to get monoalkylation with intramolecular alkylation, since its more favourable
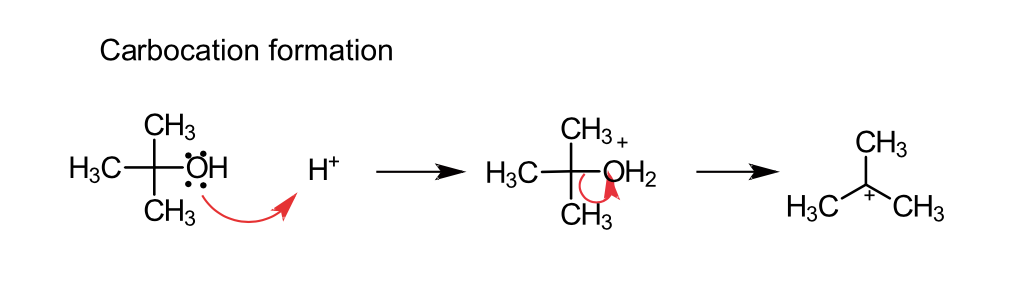
What conditions is needed for alkylation (via. alcohols)?
Requires concentrated acid (e.g H2SO4) - It helps form a carbocation
draw the mechanism of the formation of a carbocation in the alkylation via tertiary alcohols
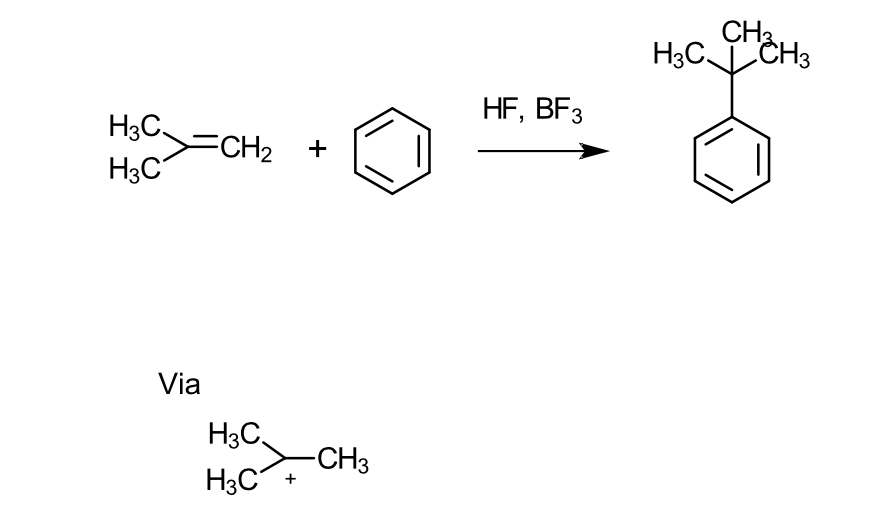
What conditions is needed for alkylation (via. alkenes (olefins)?
Requires a catalyst (e.g BF3 or HF)
what is friedal-crafts acylation?
Acyl chlorides (RCOCl) and acid anhydrides (RCCO(O)OCR) reacts with benzene
Requires molar amounts of Lewis acid catalyst
The reaction yields ketones
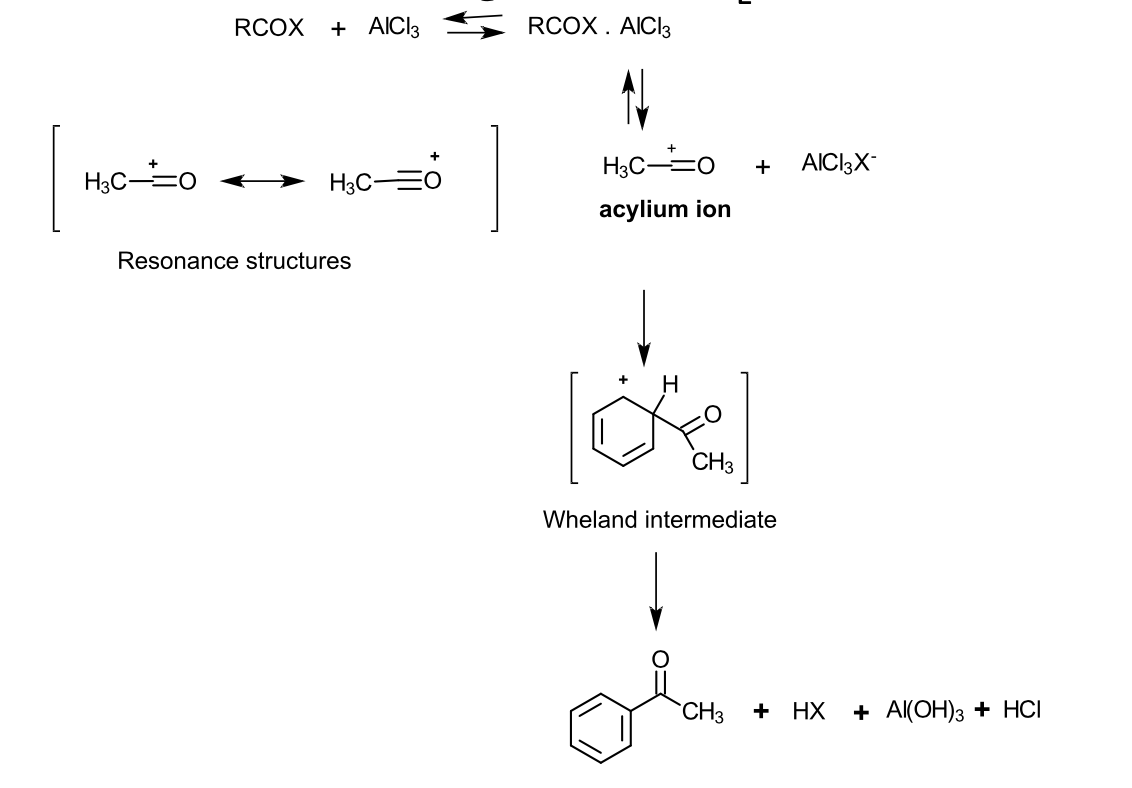
describe the mechanistic aspects of acylation (via. acylhalides):
Acyl halides (RCOX) are strong Lewis bases - they form 1:1 complexes with Lewis acids
This reaction produces a aryl ketone
The aryl ketone is more basic than the acyl chloride - this allows it to form a complex with the ketone
A full mole of Lewis acid catalyst is needed
The intermediate is called an acylium ion - reacts with benzene through electrophilic substitution
what 3 factors affect directing effects?
Electronegativity and dipole moment
Resonance effects
Hyperconjugation
what is electronegativity?
the measure of an atoms "electron attracting ability"
how does electronegativity and dipole moment affect directing effects?
Electronegativity = the measure of an atom’s "electron attracting ability"
Dipole moments is formed when there is a difference in electronegativity = which leads to permanent polar covalent bonds and dipole moments
Inductive effect (I):
depends on the polarity of the bond = the difference in electronegativity
It’s the electrostatic effect where the polarity of a bond influences electron density in adjacent bonds
The effect falls off with distance
We can have -I (electron withdrawing) or +I (electron donating) effects
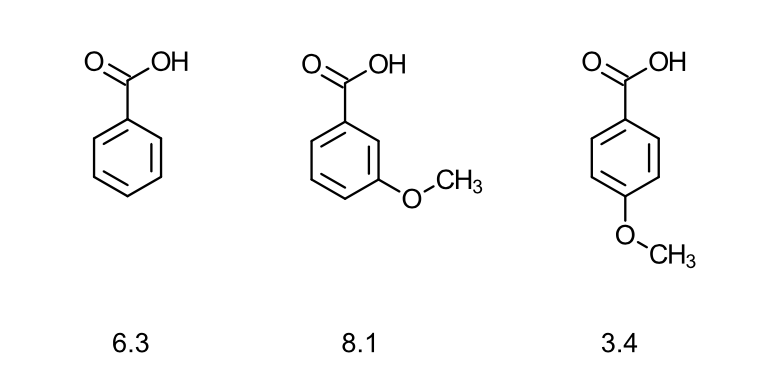
how does resonance effects (R) affect directing effects?
The meta derivative is stronger than benzoic acid, but para derivative is weaker = this is due to resonance
It forms by the conjugation of one of the lone pair electrons from the substituent with the π-system of the ring
This effect leads to a redistribution of electron density within the ring
This either increases or decreases it at specific positions (ortho, meta, para)
Resonance structures are used to visualise the changes
+R = electron DONORS
-R = electron ACCEPTORS
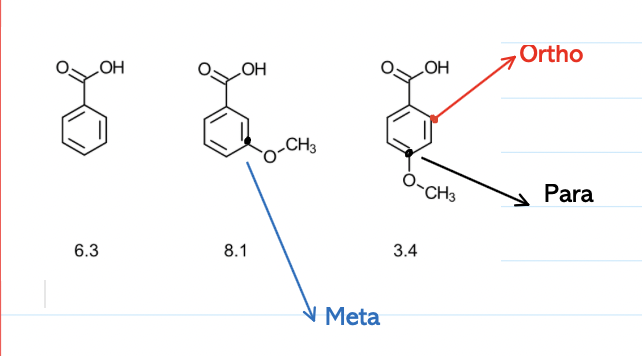
How does steric hindrance affect resonance?
Steric hindrance can impact resonance in a negative way
Bulky substituents can physically prevent a group from adopting a coplanar conformation with the aromatic ring
Lack of coplanarity disrupts the overlap needed for resonance
Therefore reduces the resonance effect
how does hyperconjugation affect directing effects?
Its the mechanism of both electron release and withdrawal
Theres C-H and C-C hyperconjugation –– C-C is the more important
tBu> iPr> ethyl> methyl