Fungi: Invasive Mold & Thermally Dimorphic Fungal Infections
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Aspergillosis
-infection caused by the genus Aspergillus (A. fumigatus is most common species)
-ubiquitous worldwide, especially in decomposing material
-humans inhale millions of spores per day
-immunity: macrophages are primary defense
-mold → hyphae are narrow and septate, branching at 45 degrees
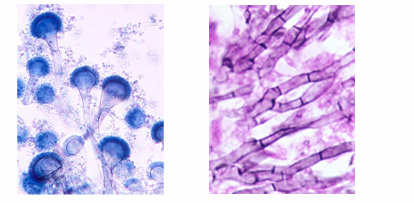
acute Aspergillosis
-pulmonary
-sinus involvement or invasion
-disseminated disease to the brain, heart, skin, or other organs
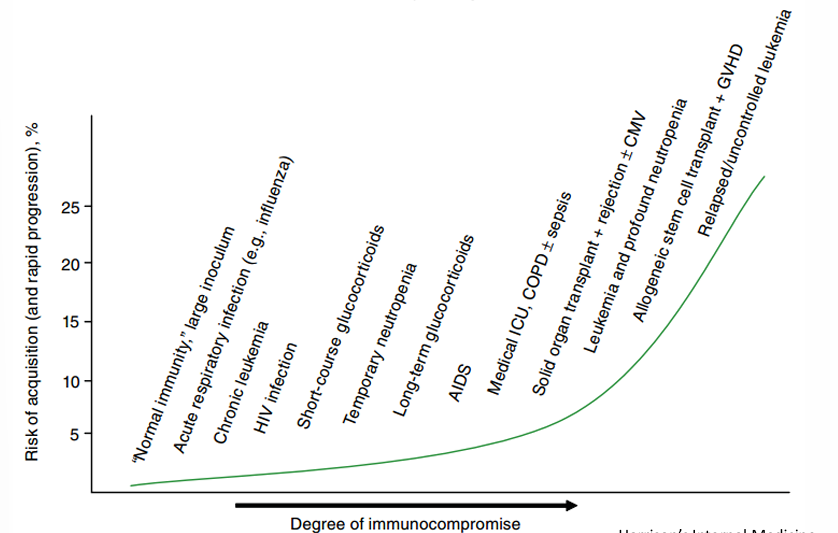
risk of Aspergillosis
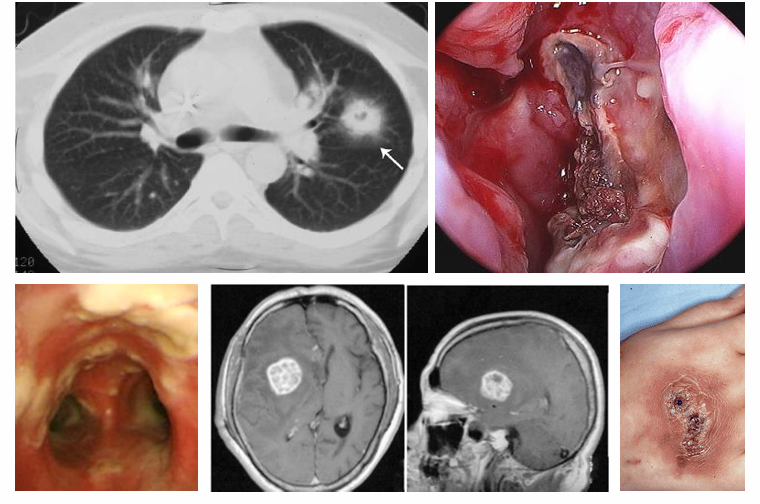
acute Aspergillosis pathogenesis
-inhalation of conidia → transfer to alveoli → bind to ECM → invasion with proteases → pneumonia
-presentation of pneumonia may vary: asymptomatic → fever, cough → hemoptysis, dyspnea, shock; 5-10% of cases also involve the sinuses (present with fever, facial pain, sinus congestion, discharge), tracheobronchial involvement also possible, disseminated disease is rare but deadly- nearly any organ can be affected (brain abscesses, endocarditis, skin)
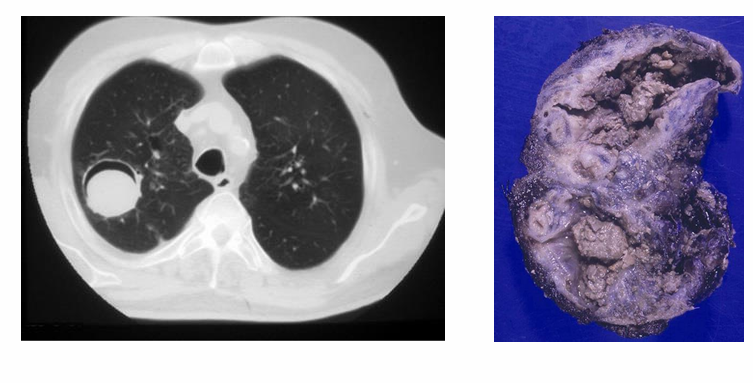
chronic Aspergillosis
-pulmonary
-sinus involvement
-Aspergilloma
chronic cavity pulmonary aspergillosis
-slow spread through cavities in patients with underlying lung disease
-presents with non-specific chronic symptoms (fever, weight loss, fatigue) → mimics TB
-cavities may have air-fluid levels or contain aspergillomas
-ongoing inflammation may progress to fibrosis
chronic aspergillus sinusitis
-slow destruction of facial sinuses
-presents with discharge, loss of smell, headache, vision changes
-may cause blindness, cavernous sinus thrombosis, or stroke
aspergilloma
-fungal balls in larger lung cavity or sinuses
-may be asymptomatic or present with cough, wheeze, fatigue
allergic aspergillosis
-allergic bronchopulmonary aspergillosis (ABPA)
-allergic sinusitis
Aspergillosis diagnostics
-histopathology (gold standard)
-cultures with poor sensitivity (10-30%)
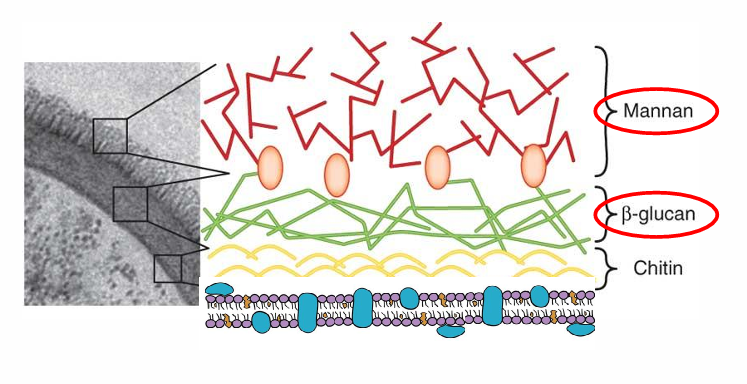
-fungal biomarkers: Galactomannan- 70% sensitive, 90% specific for invasive disease; Beta-D-Glucan (Fungitell)- more sensitive but less specific
-imaging: nodules, cavities with air-fluid levels (“halo sign” not specific; 60-70% sensitive for invasive disease)
-up to 40% of disseminated aspergillosis diagnoses are made on autopsy (!!)
aspergillus biomarkers
aspergillosis treatment
-first line: voriconazole
-other agents: Isavuconazole, posaconazole, amphotericin B, echinocandins
-combination therapy is controversial
-duration of therapy is long- 6-12 weeks minimum
-even with effective therapy, mortality of invasive aspergillosis is very high (up to 80% in stem cell transplant patients)
Mucormycosis
-highly-aggressive opportunistic fungal infection that occurs under conditions of acidosis, hyperglycemia, and iron overload- mortality is greater than 40%
-caused by molds in the order Mucorales (Rhizopus, Mucor, Rhizomucor, Cunninghamella, Absidia)
-on histology, appear as thick-walled, ribbon-like, irregular, aseptate hyphae with branches at 90-degree angles
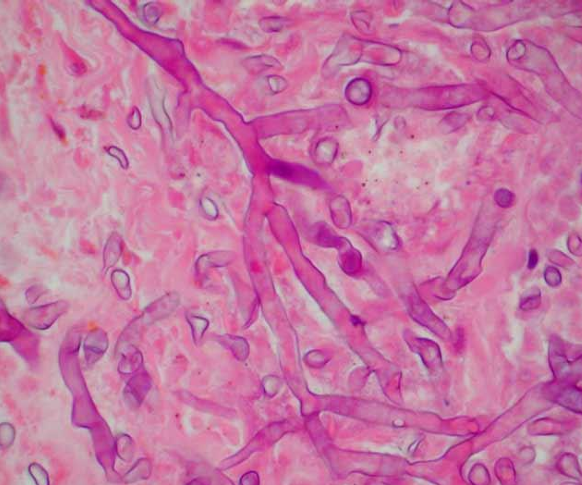
risk factors for invasive disease
1) defects in neutrophil function (diabetes, hematological malignancies, neutropenia, treatment with glucocorticoids)
2) elevated serum iron levels (ESRD, iron chelator therapy)
3) acidosis (critical illness, DKA)
4) hyperglycemia (DKA)
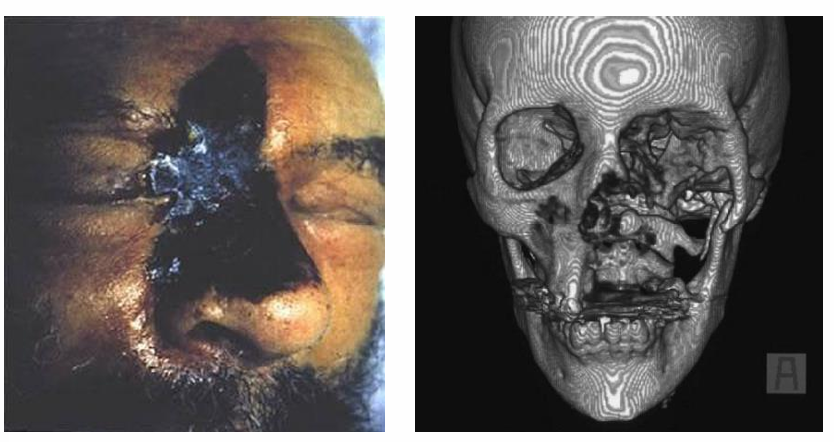
Mucormycosis clinical manifestations- rhinocerebral
-common in uncontrolled DM or DKA
-presents with facial/eye pain, numbness, soft tissue swelling
-vision changes and altered mental status may also occur
-may be complicated by bone destruction, invasion into the cavernous sinus, and invasion into the CNS
-exam may reveal erythema/edema which progress to eschar formation and necrosis
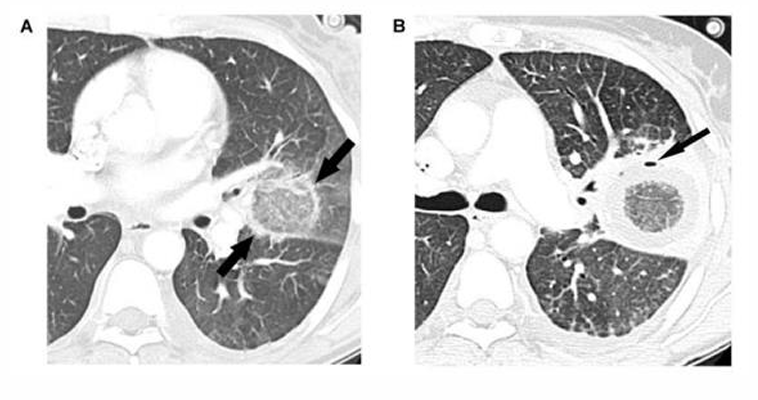
Mucormycosis clinical manifestations- pulmonary
-second most common
-presents with cough, dyspnea, chest pain, and severe hemoptysis
-CXR shows nodular consolidations
-compared to Aspergillus, there are often >10 nodules seen in mucormycosis; other findings include pleural effusions and the “reverse halo sign”
mucormycosis clinical manifestations- GI
-mainly in neonates
-presents as brisk GI bleed ± a fungating mass on endoscopy
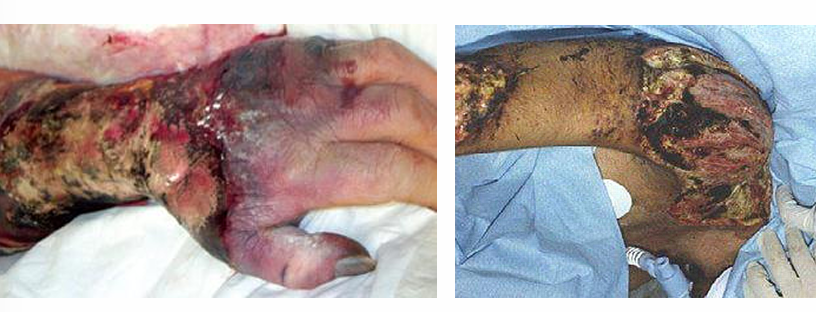
mucormycosis clinical manifestations- cutaneous
-often in immunologically normal individuals
-result of direct inoculation of fungal into skin
-source often soil although nosocomial exposures have been reported
-from the skin, fungus invades through muscle, fascia, and bone
-while aggressive surgical therapy can be curative, rapidly progressive infection has >80% mortality
mucormycosis diagnosis
-as the condition is rapidly progressive, clinical suspicion should guide therapy
-histopathology is gold standard
-culture: 50% sensitive (but takes too long)
-biomarkers (galactomannan and beta-D-glucan) generally negative
-CT and MR imaging may show opacification of sinuses and bony erosion; not specific for mucor
mucormycosis treatment
-as thrombosis and necrosis impair antifungal treatment, surgical management is essential!!
-factors that contribute to survival:
1) early diagnosis
2) reversal of risk factors (correction of acidosis, hyperglycemia)
3) emergent surgical debridement
4) prompt antifungal treatment: amphotericin B gold standard, posaconazole and isavuconazole also have activity and are occasionally used as “step-down” therapy, voriconazole has no coverage
5) routine follow-up imaging to assess for clinical changes
endemic dimorphic fungal infections defining features
-can cause invasive disease in immunologically-normal hosts
-are geographically restricted
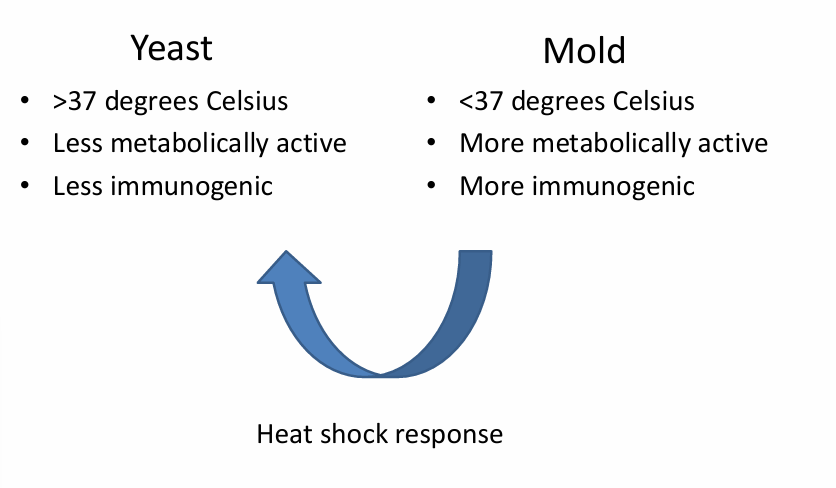
-thermally dimorphic (mold in nature, yeast in host)
dimorphic (endemic) fungi
Histoplasma capsulatum
-grows in moist soil enriched with bird and bat feces
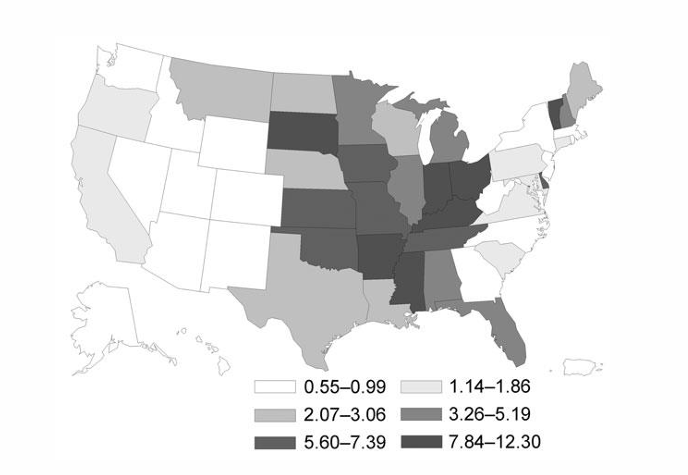
-the “capsulatum” variant is endemic in the Ohio and Mississippi River Valleys
-high risk activities: construction work, spelunking, cutting down trees, cleaning chicken coops
-grows as mycelia (mold) in the soil that produces microconidia (2-4 microns) that become aerosolized and travel to alveoli
Histoplasmosis clinical manifestations
1) asymptomatic histoplasmosis: most healthy individuals
2) acute histoplasmosis: flu-like syndrome (fevers, chills, headache, muscle aches, cough, dyspnea, chest pain) 2-4 weeks after exposure- may have autoimmune features (arthritis, erythema, nodosum) or pericarditis (uncommon); rare complications- respiratory failure, fibrosing mediastinitis
3) chronic cavitary histoplasmosis: patients with structural lung disease (ILD, COPD)
4) disseminated histoplasmosis: immunocompromised patients- acute sepsis syndrome, respiratory failure, coagulopathies, adrenal insufficiency, multi-organ dysfunction
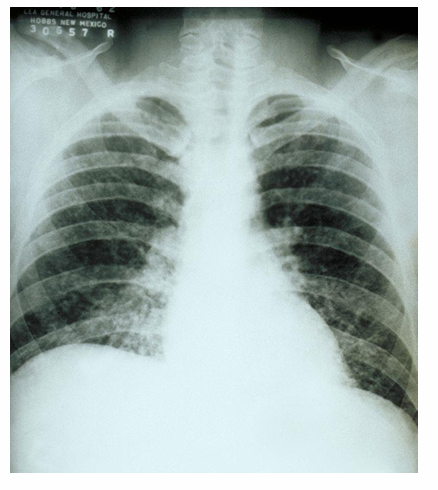
-acute histoplasmosis
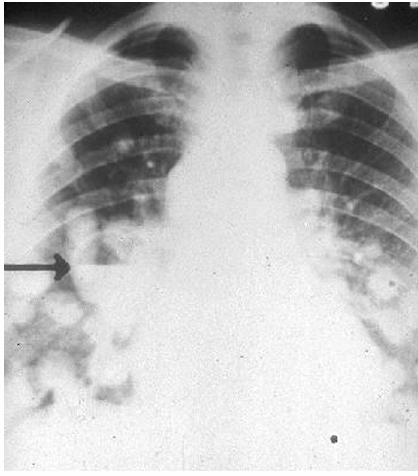
-chronic cavitary histoplasmosis
Histoplasmosis diagnosis
-culture: 50-60% sensitive in disseminated infection, less sensitive in localized; respiratory and blood cultures are most common- bone marrow culture most sensitive
-fungal stains are 50% sensitive in disseminated disease
-histoplasma urine and serum antigens are 80-95% sensitive
Histoplasmosis treatment
-mild acute histoplasmosis: Itraconazole for 12 weeks
-chronic cavitary histoplasmosis: Itraconazole for 12 months
-severe, neurological, or disseminated histoplasmosis: amphotericin B for 6 weeks followed by Itraconazole for 12 months
Coccidioidomycosis
-thermally dimorphic fungi present in soil
-two medically important species: C. immitis and C. posadasii
-infection occurs with direct exposure to previously undisturbed soil (construction work, earthquakes, archaeological excavations; increased cases following rainy seasons)
-in US, endemic in Central California and desert Southwest, also present in Mexico, Central America, South America
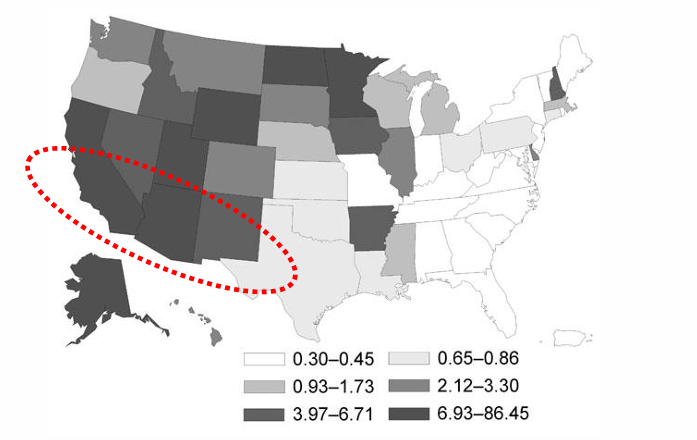
Coccidioidomycosis in soil
-exists as a mycelia
-when contacted, these release arthroconidia (2-4 microns) that are highly resistant to harsh environmental conditions
-aerosolize and are inhaled into the terminal bronchioles
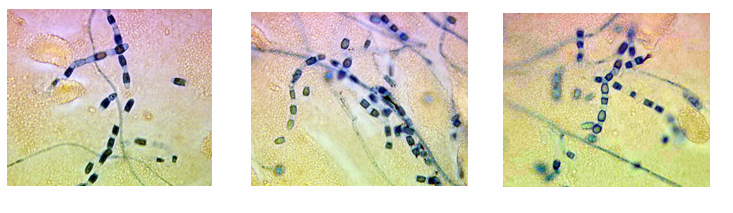
Coccidioidomycosis clinical manifestations
1) asymptomatic: 60% of exposed individuals
2) primary pulmonary: 40%, “Valley Fever”- presents 2-6 weeks after inoculation, presents similar to community-acquired pneumonia (fever, cough, etc.), CXR shows infiltrates and hilar lymphadenopathy; approximately 10% will have pleural effusions
3) chronic pulmonary: individuals with pre-existing lung disease, may resemble TB or malignancy (fever, weight loss, fatigue)
4) disseminated: immunocompromised patients (pulmonary- diffuse lung involvement, CXR infiltrates, respiratory failure; extra-pulmonary sites: skin, bone, joints, soft tissues, and meninges)
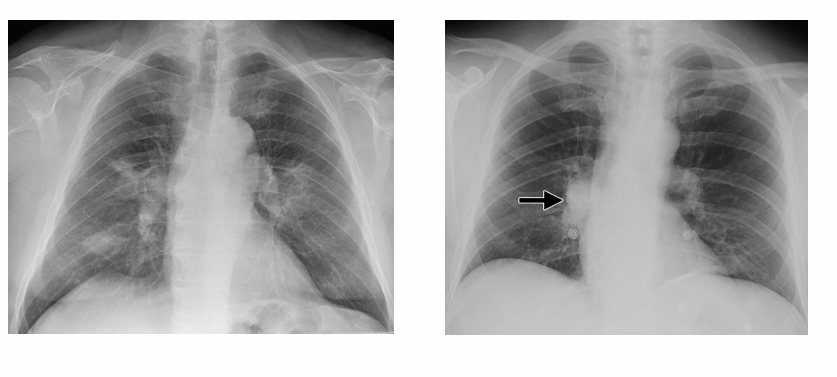
-acute pulmonary Coccidioidomycosis
extrapulmonary coccidioidomycosis
-cutaneous: 10%, erythema nodosum
-musculoskeletal: arthralgias, arthritis
Blastomycosis
-endemic thermally dimorphic yeast with microconidia that are easily aerosolized
-found in Mississippi River Valley as well as the Southeast, overlapping somewhat with the distribution of Histoplasmosis
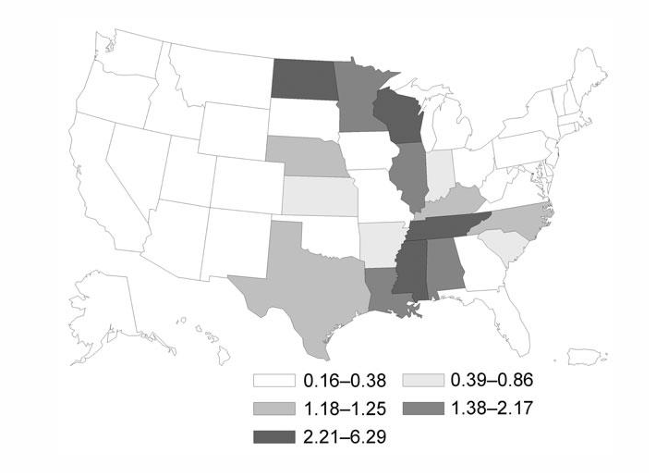
Blastomycosis outbreaks & yeast form
-outbreaks often occur in individuals with exposure to decaying wood
-yeast form is 8-15 microns (much larger than Histoplasma), have thick walls, and reproduce by broad-based budding
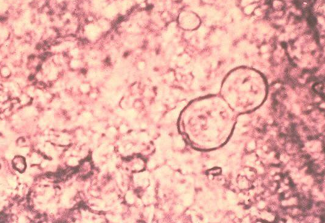
Blastomycosis diagnosis
-culture- takes up to 4 weeks
-microscopy from biopsies may have high yield
-serologies may be useful; there is no antigen
Blastomycosis treatment
-acute of chronic pulmonary blastomycosis: Itraconazole for 6-12 weeks
-severe, neurological, or disseminated blastomycosis: amphotericin B for 6 weeks followed by itraconazole for 12 months
-other azoles
take home points
