Required practical 4: Temp change.
1/9
Earn XP
Description and Tags
https://www.youtube.com/watch?v=rdI7xEq4Ew8&list=PL9IouNCPbCxX74bPfz0TGVVmyGYgMarWu&index=4
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is the practical summarised in short words.
Investigating temp cchange in exo reaction
Neutralistion reaction between hydrochloric acid and alkaline sodium hydroxide.
Add increasing volumes of sodium hydroxide to hydrochloric acid.
In each experiment, measure the max temp reached.
Whats independent, dependent and control variables.
Independ - Vol of sodium hydroxide.
Dependent variable - Max temp reached.
Control - Volume of Hcl. concentration of hcl and sodium hydroxide.
Whats first step.
Start by using measuring cylinder to measure 30cm³ of dilute hydrochloric acid.
Whats second step.
Transfer the acid to a polystyrene cup, then stand the polystyrene cup inside a beaker to stop it from falling. Then use a thermometer to measure temp of acid and record in table.
Whats third step
Use a measuring cylinder to measure 5cm³ of sodium hydroxide, and transfer this to the polystyrene cup, and at this point we fit a plastic lid to the cup. and place a thermometer through the hole in the lid.
Whats 4th step
Use the thermometer to gently stir the solution.
Because its an exo reaction , it will release energy, this means temp of the solution will increase.
Whats 5th step
Look carefully at the temp rise on the thermometer.
When the reading on the thermometer stops changing, we record the highest temp reached.
Whats 6th step
Rinse out and dry the polystyrene cup, and repeat the whole experiment using 10cm³ of sodium hydroxide, keep carrying out same experiment and increasing volume of sodium hydroxide by 5cm³, until we reach a max of 40cm³
Repeat whole experiment one more time so that theres 2 sets of results, and a mean can be calculated for each volume of sodium hydroxide solution., and graph results.
What does the graph show.
As we increase volume of sodium hydroxide solutions, the max temp reached increases.
This s because when we add more particles of sodium hydroxide they react with hydrochloric acid.
This is exo reaction , so more energy is released and max temp reached increases.
However at certain volume, max temp decreases, this is because too much sodium hydroxide added, and not enough hydrochloric acid.
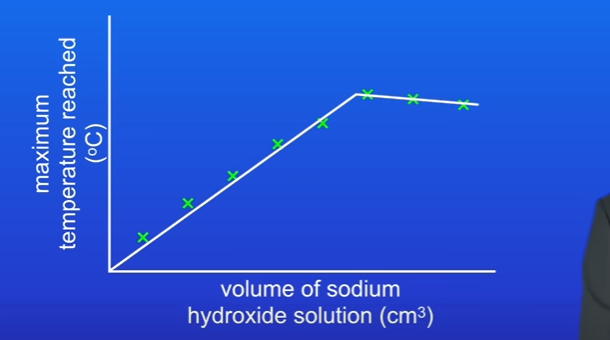
Why does max temp decrease from a certain point.
Greater volume of solution is being added each experiment
So energy released is spread out into a greater volume
So , when we add large volumes of sodium hydroxide, the max temp decreases