Kinetic Theory and Internal Energy
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
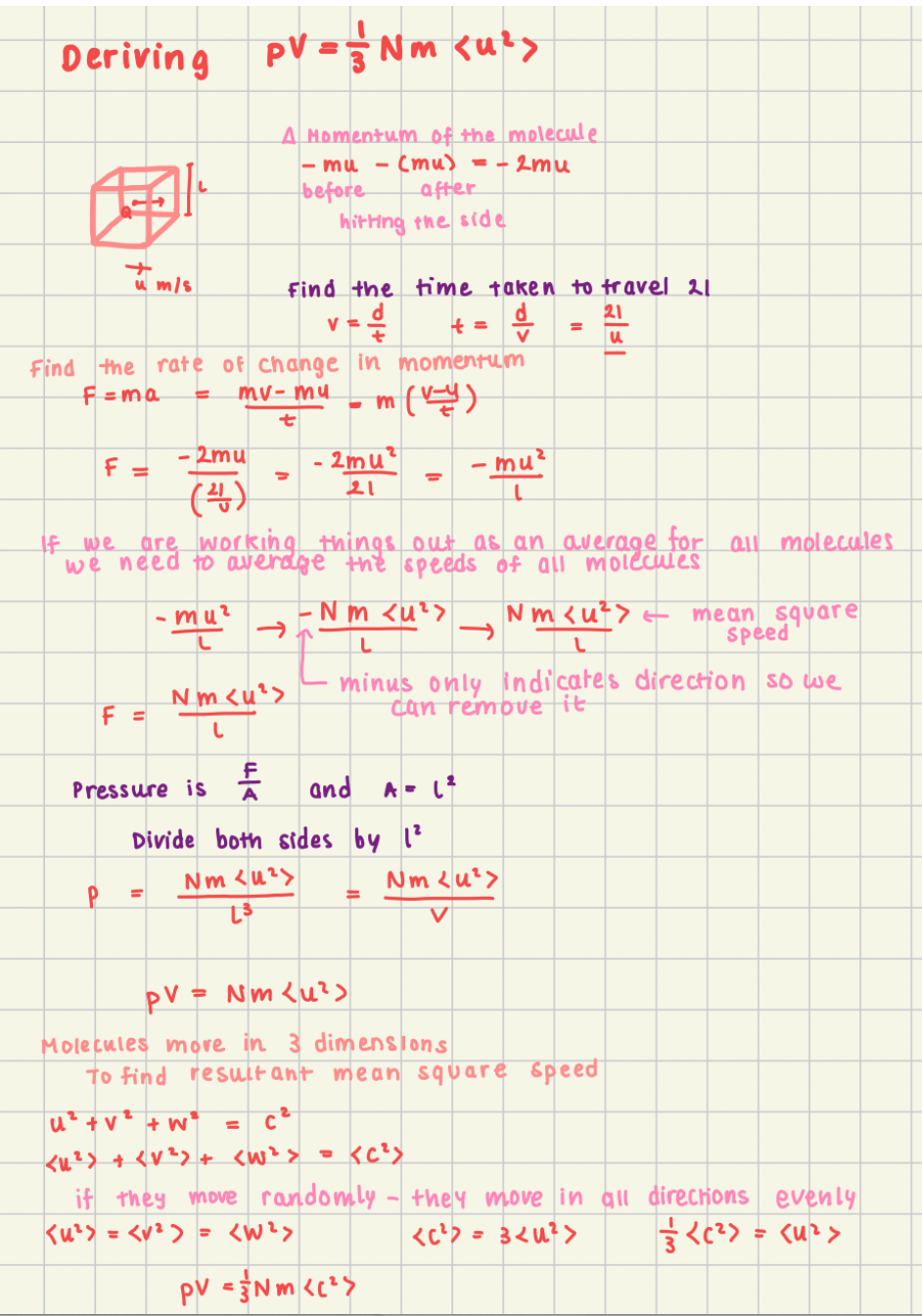
How do you derive the equation pV=1/3Nm<u²>
.
root mean squared
The average square of all molecules = <c^2>
Root mean squared = root of <c^2>
What is the use of root mean squared?
To find the typical speed of the molecules
Equation of the Internal energy of an ideal gas of an individual molecule
In ideal gases internal energy = kinetic energy only
1/2m<c^2> for an individual molecule
ideal gas equation pV=NkT
and pV=1/3Nm<c^2>
combining them you get 1/2m<c^2> =3/2kT
What does the equation 1/2m<c^2>=3/2kT show?
internal energy of an idea gas is proportional to its absolute temperature
so
the rise in absolute temperature increases the kinetic energy of each molecule, causing a rise in internal energy