General Chemistry I: Exam 1
1/32
Earn XP
Description and Tags
Content on Chapters 1-3 of the textbook
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
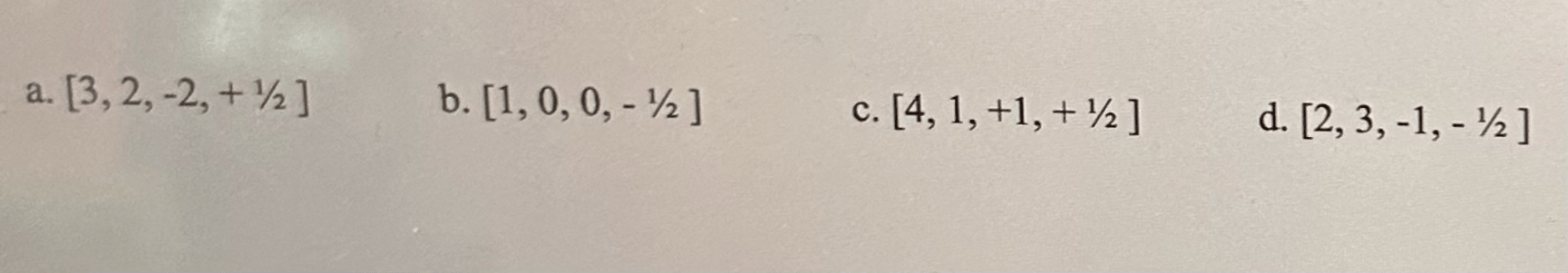
What is this problem asking about?
Quantum Numbers
How many quantum numbers are there in total in a set of them?
4
what does the first quantum number tell you?
What row of the periodic table the electron’s atom is in (must be 1-7)
What does the second quantum number tell you?
which orbital of the atom the electron is in (must be 0-3)
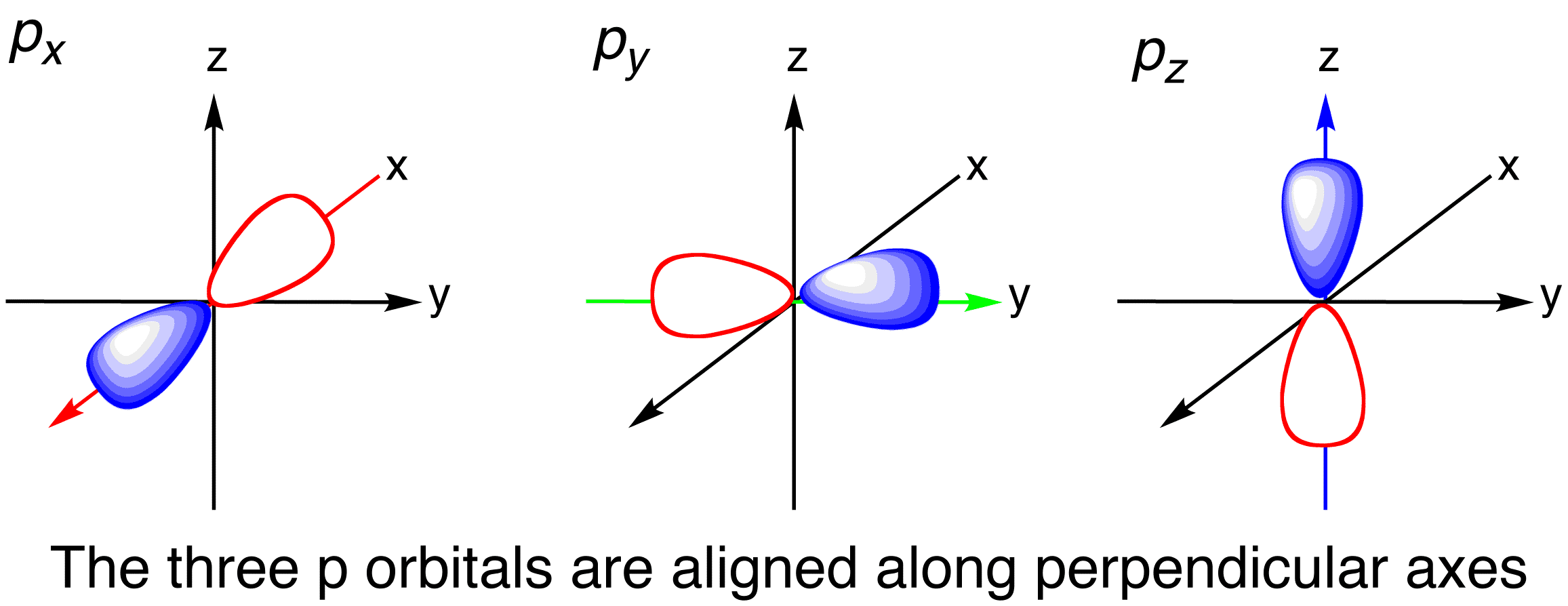
What does the third quantum number tell you?
must be a whole number, must be greater than or equal to -3, and less than or equal to 3
if the second quantum number is 0, the third quantum number must always also be 0
What does the fourth quantum number tell you?
must be ½ or -½
what is the formula for Hz?
Hz = 1/s OR Hz = 1/T (T being unit of time)
what symbol represents “frequency”?
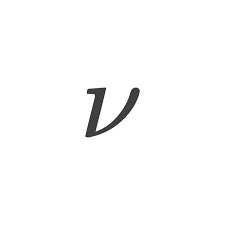
what symbol represents the speed of light?
c
what symbol represents wavelength?
lamda
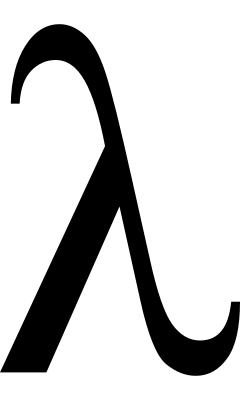
what is the difference between the two n values in equations about orbitals?
one is ni (initial orbital) and one is nf (final orbital)
initial n (ni) will always be the higher #
pronounced “n sub i” or “n sub f”
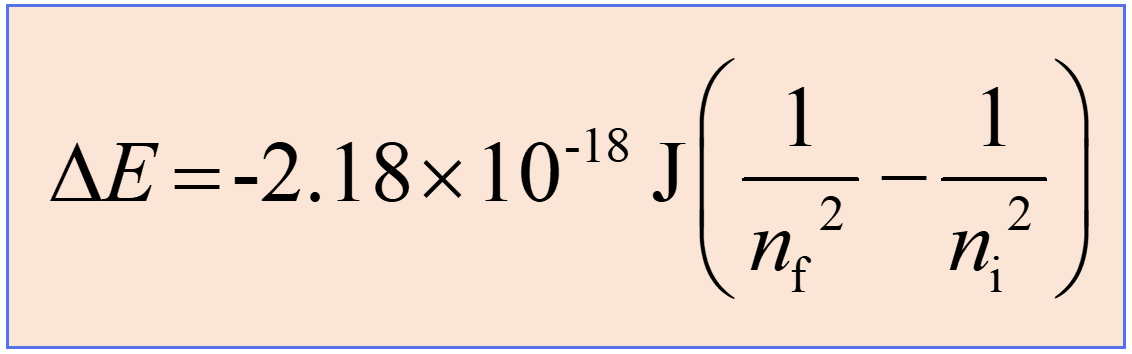
What information does this equation give you?
this is the Rydberg equation (on the formula sheet, but not by name)
tells you the amount of energy in the photon released by the electron switching orbitals
the bigger the orbital drop…
the smaller the wavelength
nano
n
109 (nine sounds like nano)
Do you get your sig figs from the unique values given in the prob (like frequency or wavelength) or from constants like c (speed of light)?
unique values
n
nano
10-9
hecto
h
102
h
hecto
102
kilo
k
103
k
kilo
103
mega
M
106
M
mega
106
giga
G
109
centi
c
10-2
c
centi
10-2
milli
m
10-3
m
milli
10-3
micro
µ
10-6
µ
micro
10-6
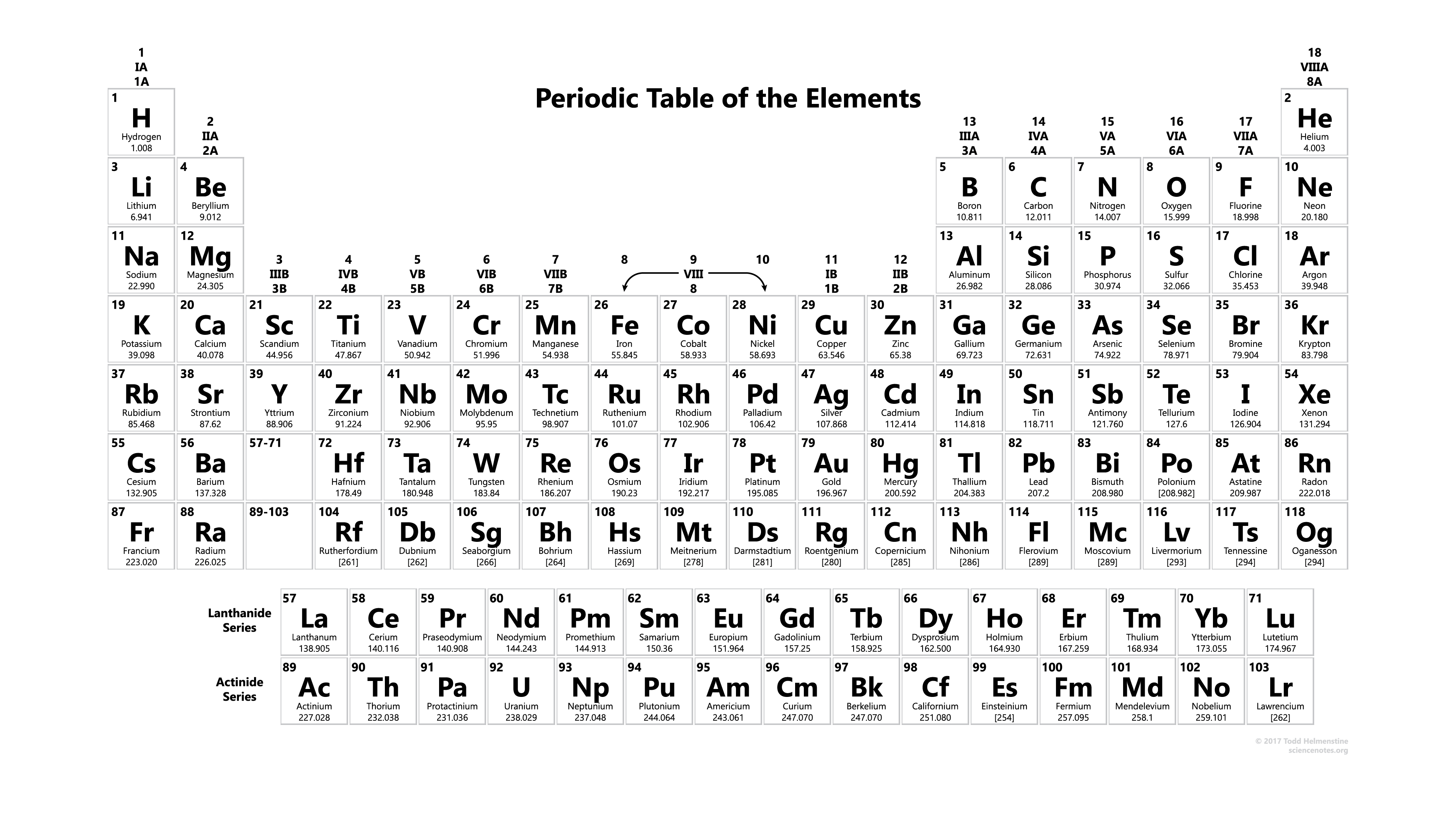
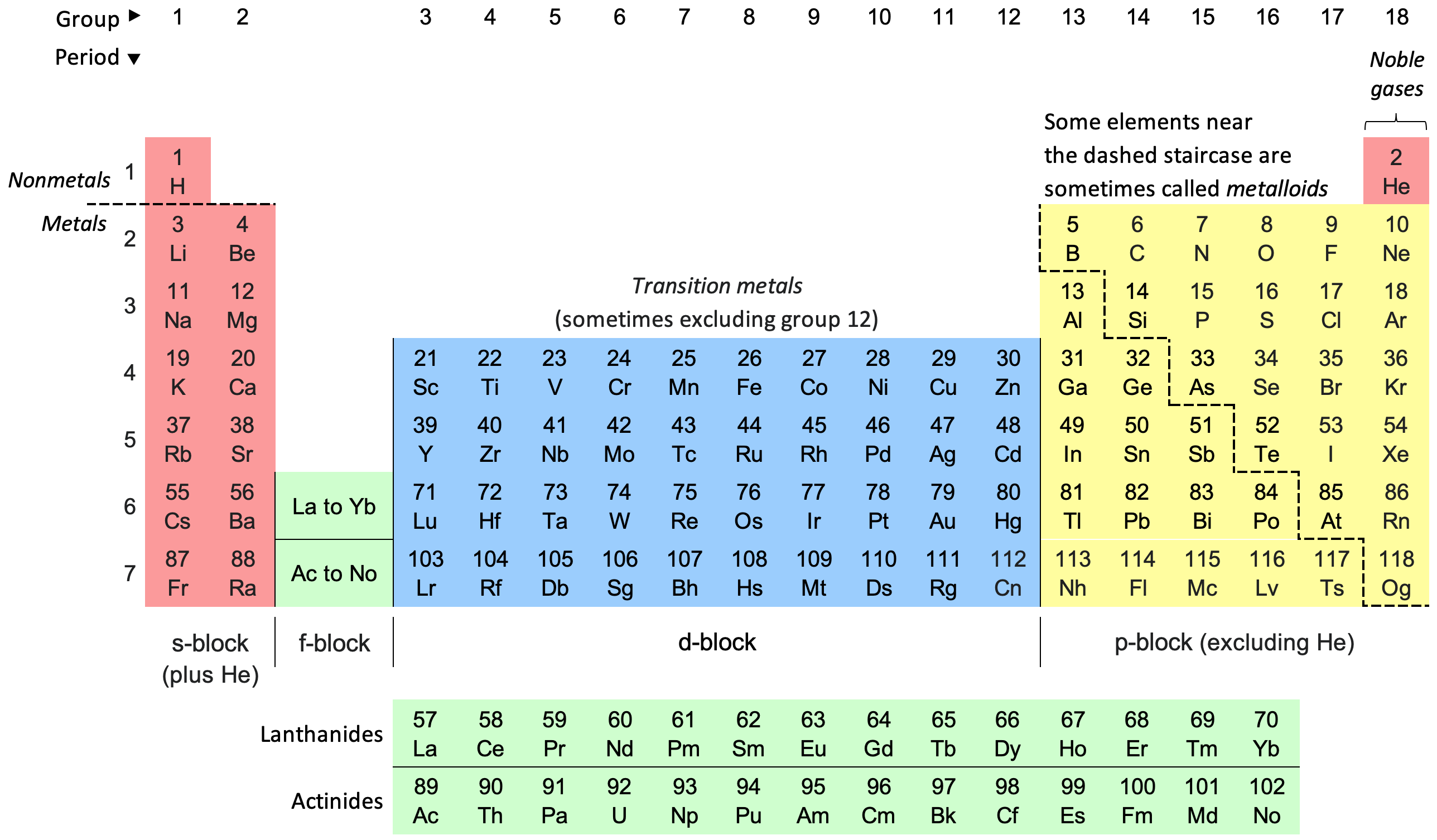
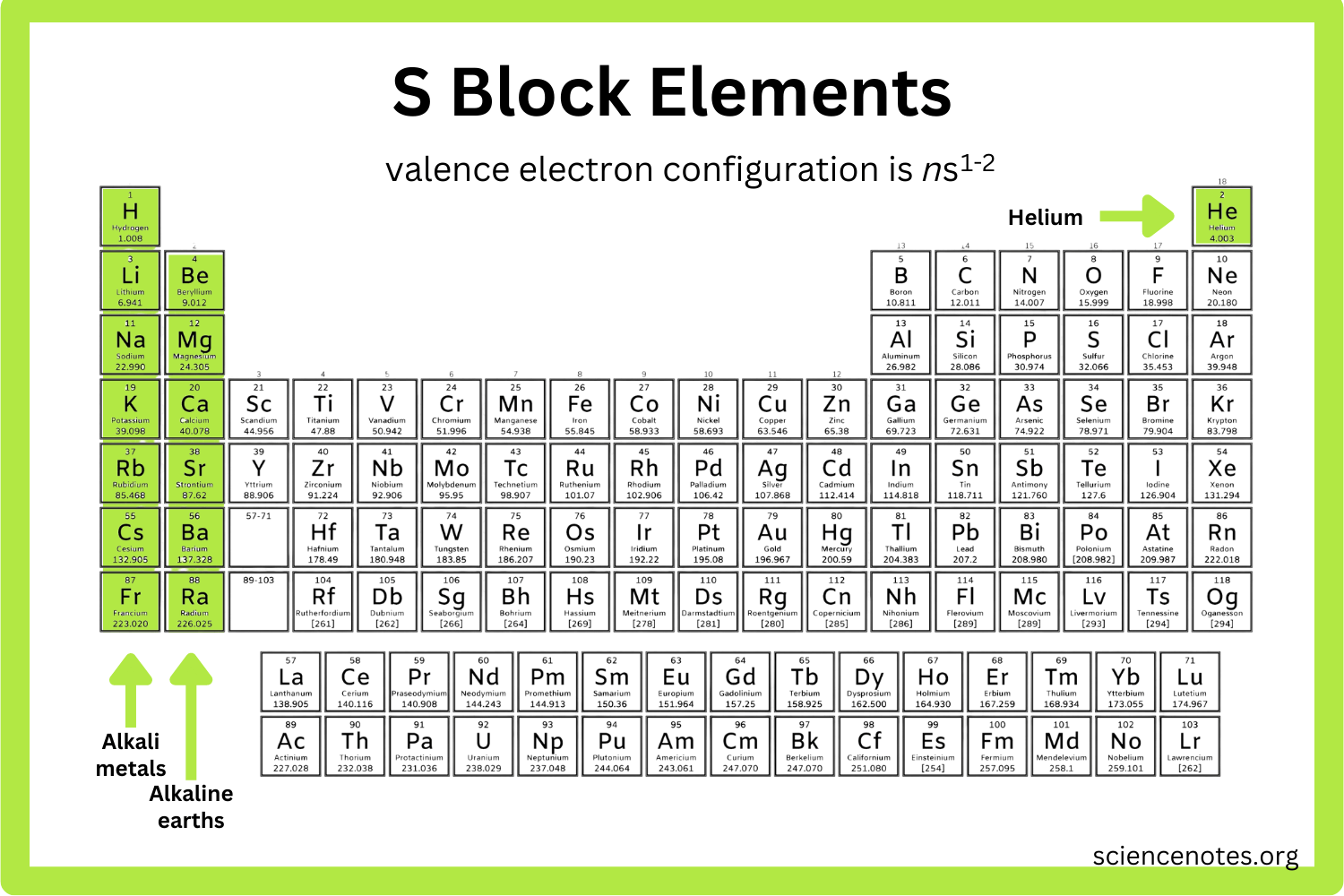
where is the s block on the periodic table?
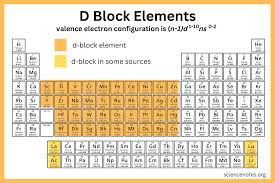
where is the d block on the periodic table?
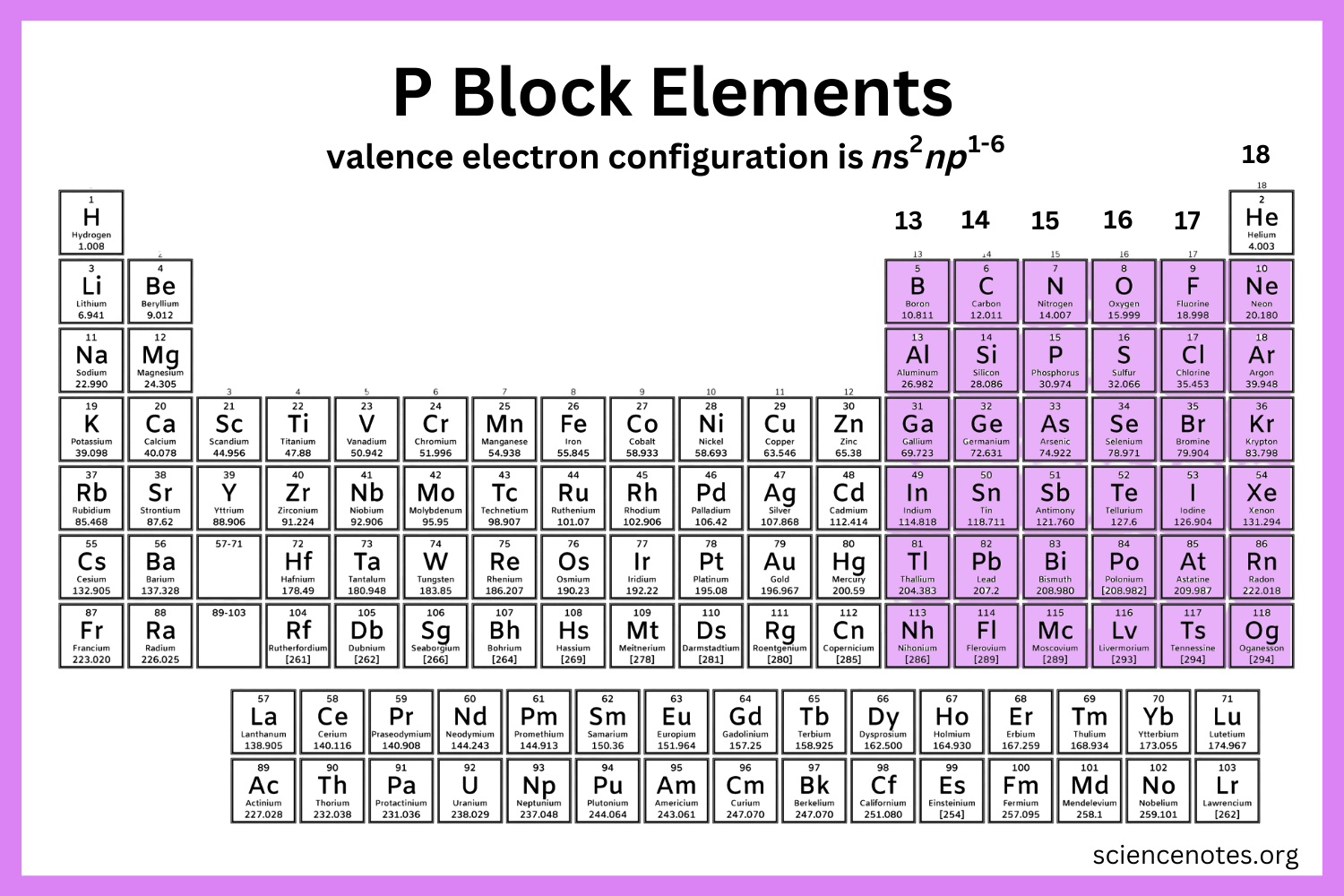
where is the p block on the periodic table?
where is the f block on the periodic table?