Dyslipidemia, Lipoproteins, and Pharmacology: A Comprehensive Review
1/156
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
157 Terms
What are the main components of lipoproteins?
Protein combined with:
Cholesterol
Triglycerides (TG)
phospholipids combined
Why do lipoproteins need to transport cholesterol and triglycerides?
Cholesterol and triglycerides are insoluble in plasma and require lipoproteins for transport.
What are the major classes of lipoproteins?
LDL (Low-Density Lipoproteins)
HDL (High-Density Lipoproteins)
VLDL (Very Low-Density Lipoproteins)
IDL (Intermediate Density Lipoprotein).
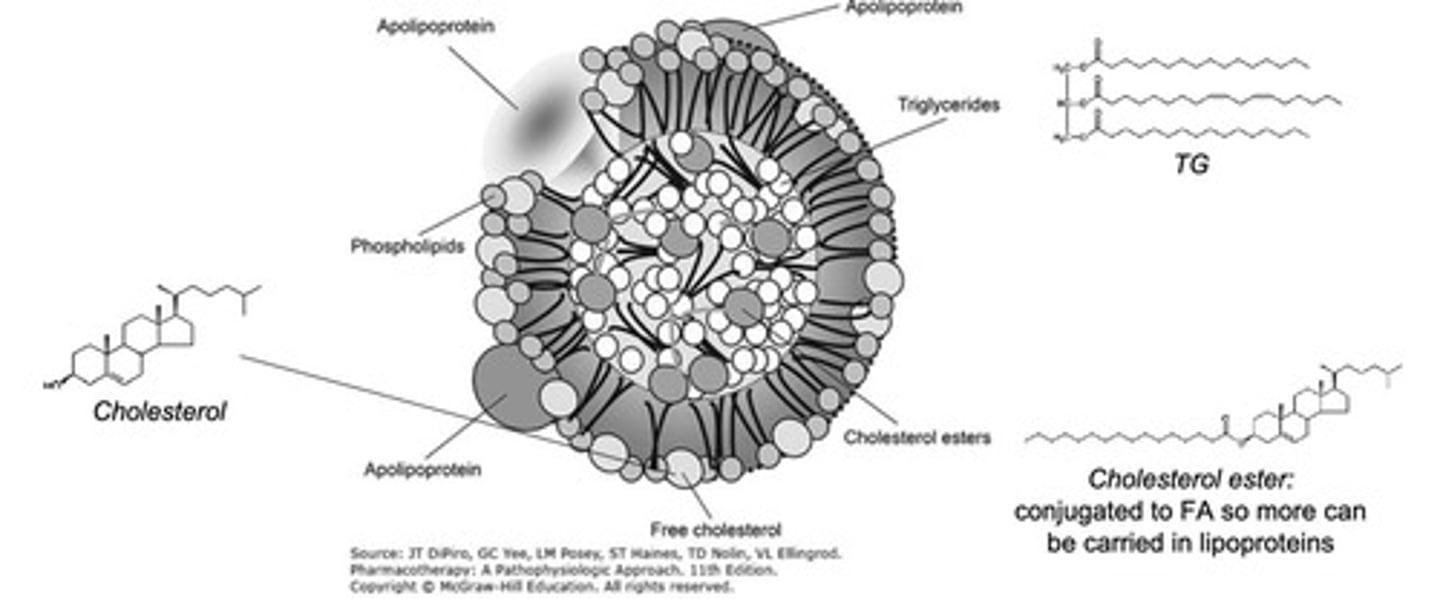
What is the role of cholesterol esters (CE) and triglycerides (TGs) in lipoproteins?
CE and TG are core components inside lipoproteins due to their lipophilic properties
Apolipoproteins are on the surface to interact with the environment.
What are the risk factors associated with dyslipidemia?
increased risk for Atherosclerotic Cardiovascular Disease (ASCVD)
nonfatal myocardial infarction (MI)
coronary heart disease (CHD)
stroke
What is Primary or Familial Dyslipidemia?
A genetic condition leading to increased risk of premature ASCVD due to significant elevations in cholesterol levels.
What causes Secondary or Acquired Dyslipidemia?
lifestyle choices
disease states
medications
diet
What defines Familial Hyperchylomicronemia?
Massive fasting hyperchylomicronemia with greatly elevated serum TG levels → often due to a deficiency of lipoprotein lipase (LPL).
What defines Familial Hypercholesterolemia?
Characterized by elevated LDL and normal VLDL levels due to a block in LDL degradation, leading to increased serum cholesterol.
What defines Familial Combined Hyperlipidemia?
Elevated VLDL and LDL levels resulting in increased serum TG and cholesterol levels → caused by overproduction of VLDL by the liver.
What is Familial Hypertriglyceridemia?
Increased VLDL levels with normal or decreased LDL levels, resulting in elevated circulating TG levels, often seen in obese and diabetic patients.
What is the role of bile acids in fat digestion?
Bile acids help break down dietary fats into micelles, facilitating the formation of free fatty acids (FFA).
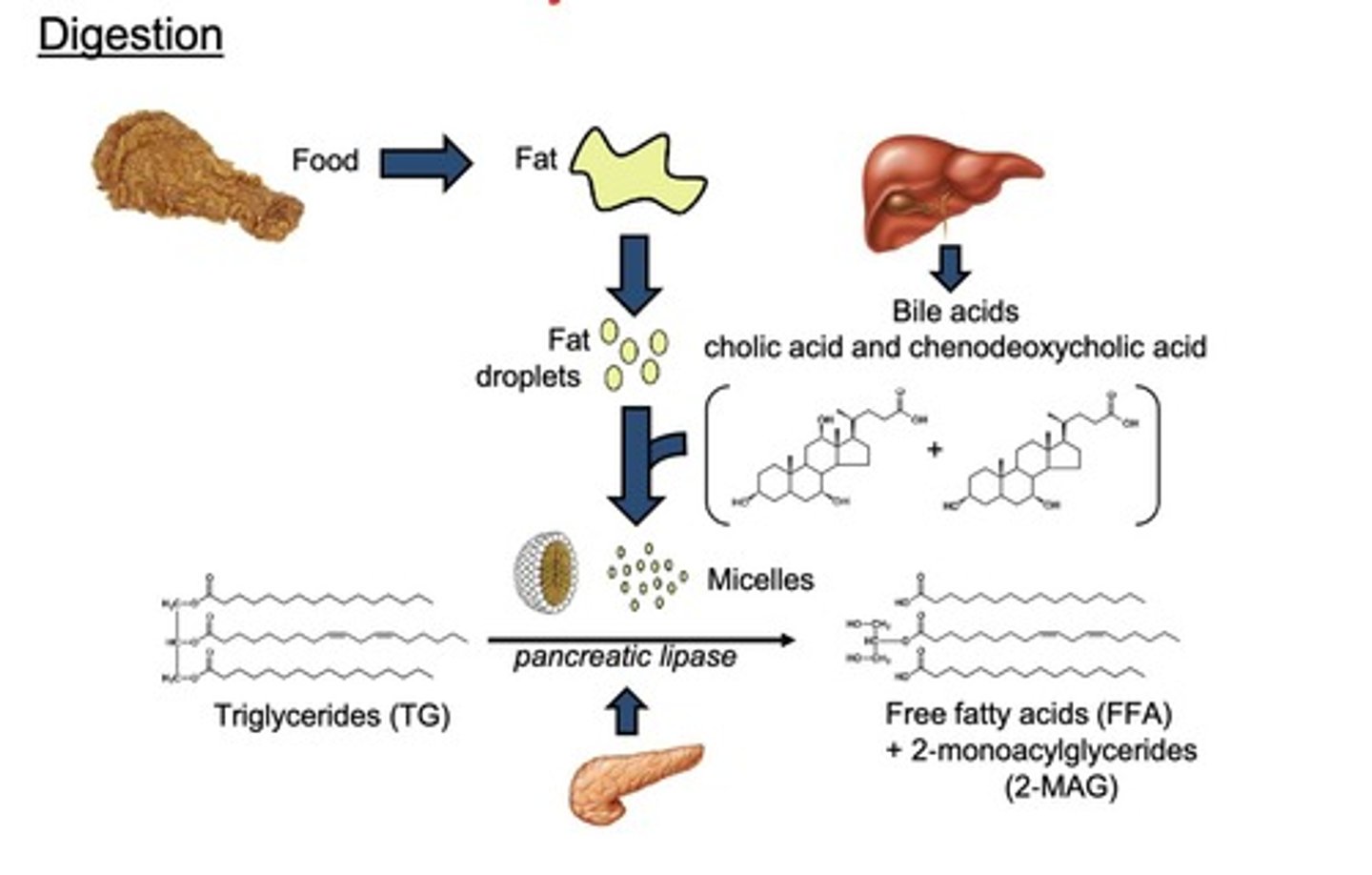
What enzyme is responsible for breaking down triglycerides?
Pancreatic lipase.
What are the components of micelles?
free fatty acids (FFA)
2-monoacylglycerides (2-MAG)
cholesterol
bile acids.
What are the component percentages of chylomicrons?
85-90% triglycerides
5-10% phospholipids
1-3% cholesterol esters (CE)
1-2% apolipoproteins (Apo) E & C2.
How do chylomicron remnants contribute to lipid metabolism?
They bind to LDL receptors on the liver to synthesize cholesterol or transport fatty acids for storage.
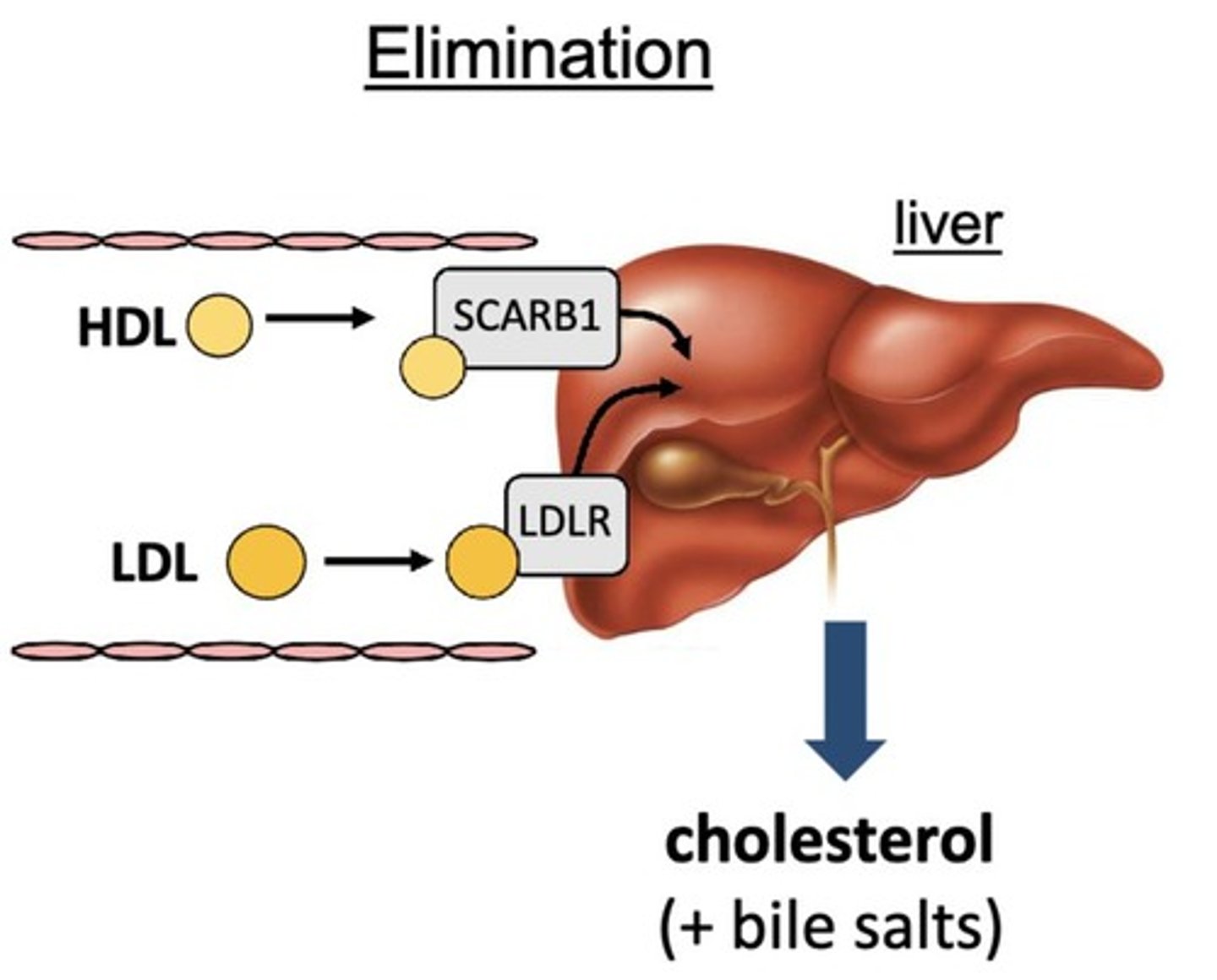
What is the function of LDL receptors (LDL-R)?
LDL-R on the liver binds to LDL or chylomicron remnants for utilization in cholesterol synthesis.
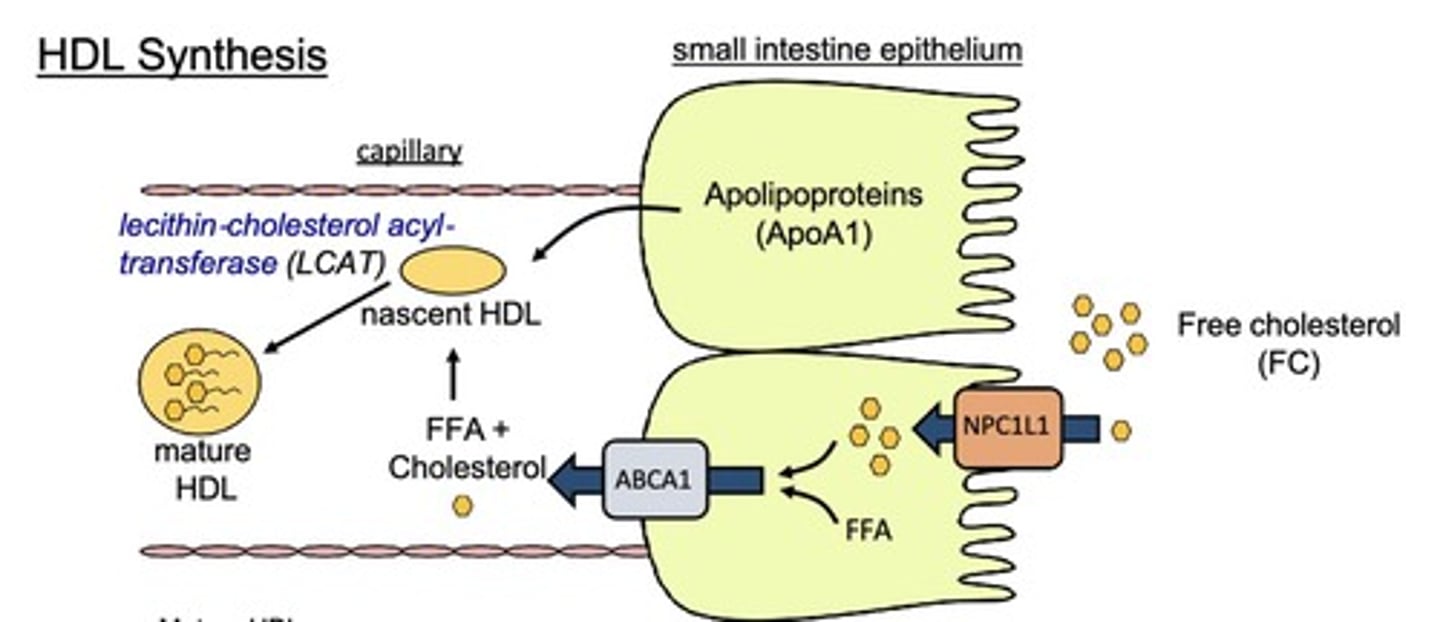
What is the process of HDL synthesis?
ApoA1 helps form nascent HDL, which matures with the addition of FFA and Chol, aided by lecithin-cholesterol acyltransferase (LCAT).
What is the role of lipoprotein lipase (LPL)?
LPL converts VLDL and IDL into FFA for storage in adipose tissue.
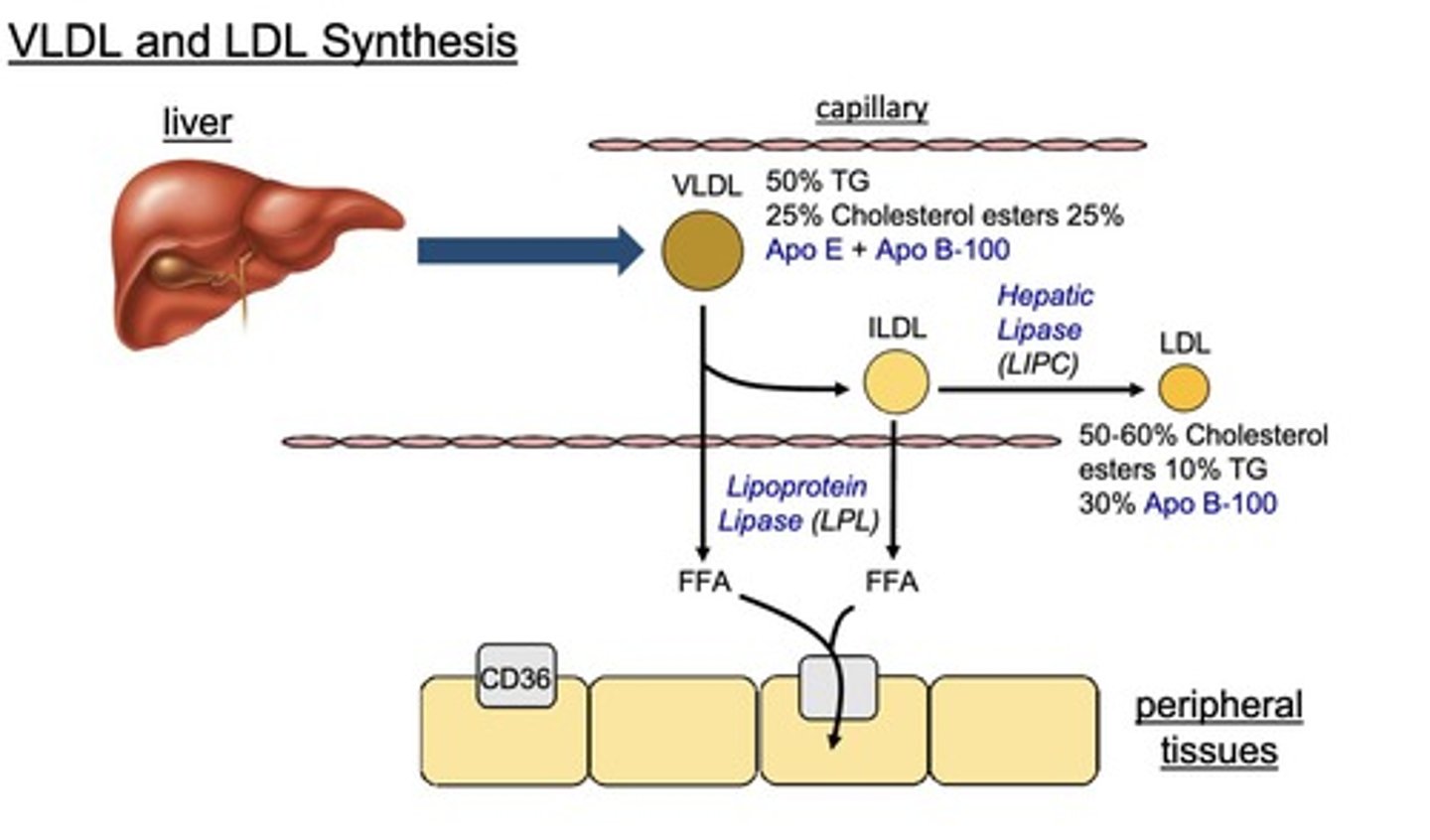
How does LDL undergo cellular uptake?
LDL binds to LDLR on peripheral tissues, leading to endocytosis and lipolysis to form free cholesterol and fatty acids.
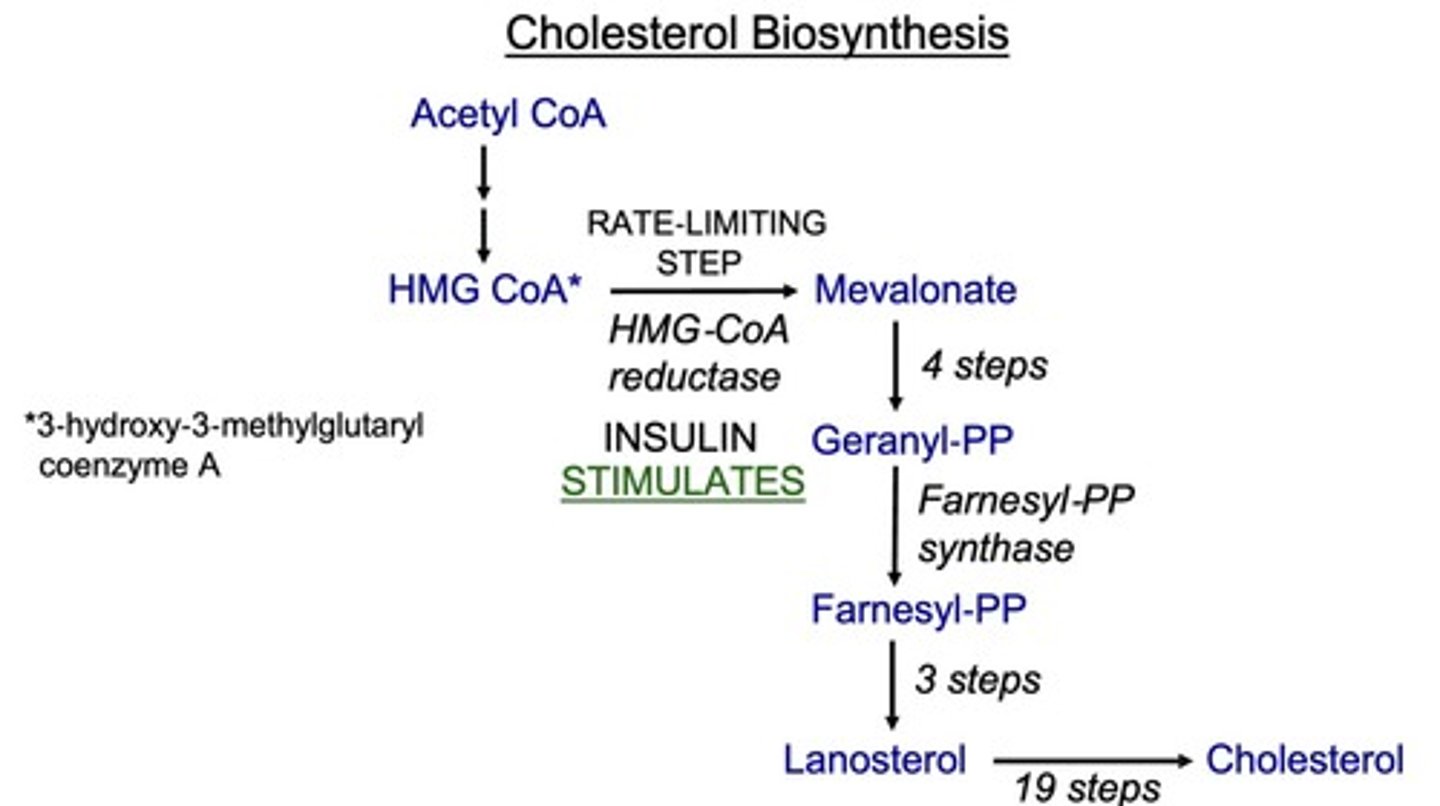
What is the first step of cholesterol synthesis?
the conversion of HMG-CoA to mevalonate by HMG-CoA reductase.
Why is LDL considered 'bad' cholesterol?
LDL can become oxidized in circulation, leading to foam cell formation and promoting atherosclerosis.
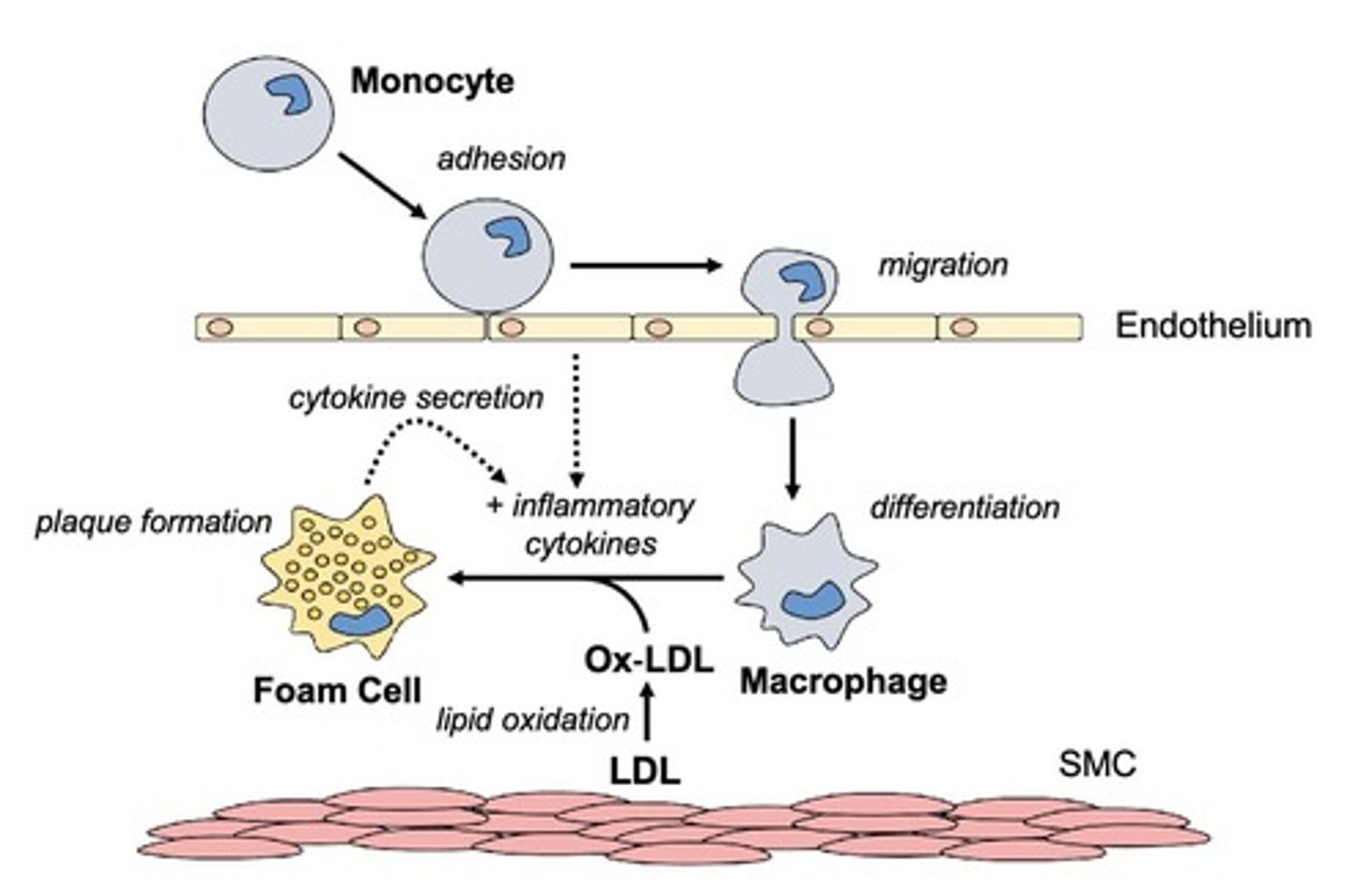
What role do macrophages play in atherosclerosis?
Macrophages engulf oxidized LDL to form foam cells, contributing to plaque formation in arteries.
What triggers the conversion of LDL to oxidized LDL?
Oxidants (-OH, O2, etc.) and enzymes such as myeloperoxidase (MPO) convert LDL to oxidized LDL, which signals macrophages to remove excess LDL.
What is the composition of a thrombus?
platelets
coagulation factors
fibrin
side-note: can grow quickly to occlude arteries.
What enzymes are responsible for the removal of oxidized LDL?
Paroxonase 1 (PON1)
platelet activating factor acetyl hydrolase
(PAF-AH).
What is the role of CD36 in macrophages?
CD36 allows for ox-LDL to be engulfed/internalized by the macrophage, leading to the formation of foam cells.
What is the mechanism of action of statins?
they are competitive inhibitors of HMG-CoA reductase, blocking the conversion of HMG-CoA to mevalonate, which decreases cholesterol synthesis.
What is the rate-limiting step of cholesterol synthesis?
The conversion of HMG-CoA to mevalonate by HMG-CoA reductase.
What are the effects of statins on LDL-C levels?
decrease LDL-C by 50-60%
decrease triglyceride levels by 10-20%
increase HDL levels by 5-15%.
What are the indications for statin use?
they are indicated for dyslipidemia, primary prevention of cardiovascular disease, and secondary prevention of cardiovascular events.
Name two high-intensity statins and their doses.
Atorvastatin (Lipitor): 40-80 mg
Rosuvastatin (Crestor): 20-40 mg
What are the short-lived statins?
Fluvastatin (1-3 hr)
Lovastatin (3-4 hr)
Pravastatin (1-3 hr)
Simvastatin (2-3 hr).
What are the long-lived statins?
Atorvastatin (14-19 hr)
Pitavastatin (12 hr)
Rosuvastatin (20 hr).
What is a common adverse effect of statins?
Myalgia with normal CK levels
myopathy with moderate CK elevation
[rare] rhabdomyolysis with very high CK levels.
How can CoQ10 supplements help with statin-induced myalgia?
may alleviate myalgia by improving mitochondrial function in muscle fibers.
Which statins are metabolized by CYP3A4?
Lovastatin
Simvastatin
Atorvastatin
What are common drug interactions with statins?
Azole antifungals (e.g., Itraconazole)
macrolides (e.g., Erythromycin, Clarithromycin),
HIV protease inhibitors (e.g., Ritonavir)
grapefruit juice
What is the mechanism of PCSK9 inhibitors?
PCSK9 inhibitors block PCSK9 from degrading LDL receptors, increasing LDL receptor levels and decreasing LDL-C.
What are the effects of mAb and siRNA PCSK9 inhibitors?
mAb decreases LDL-C by 40-70%
siRNA decreases LDL-C by 50%.
What is the mechanism of MTP inhibitors?
block the conversion of triglycerides into pre-VLDL, preventing LDL-C formation.
What are the adverse effects of Lomitapide?
High incidence of hepatotoxicity
contraindicated in pregnancy
hepatic impairment.
What is the mechanism of Ezetimibe?
Ezetimibe blocks the uptake of cholesterol in the intestine → increasing LDL-R expression and decreasing LDL-C.
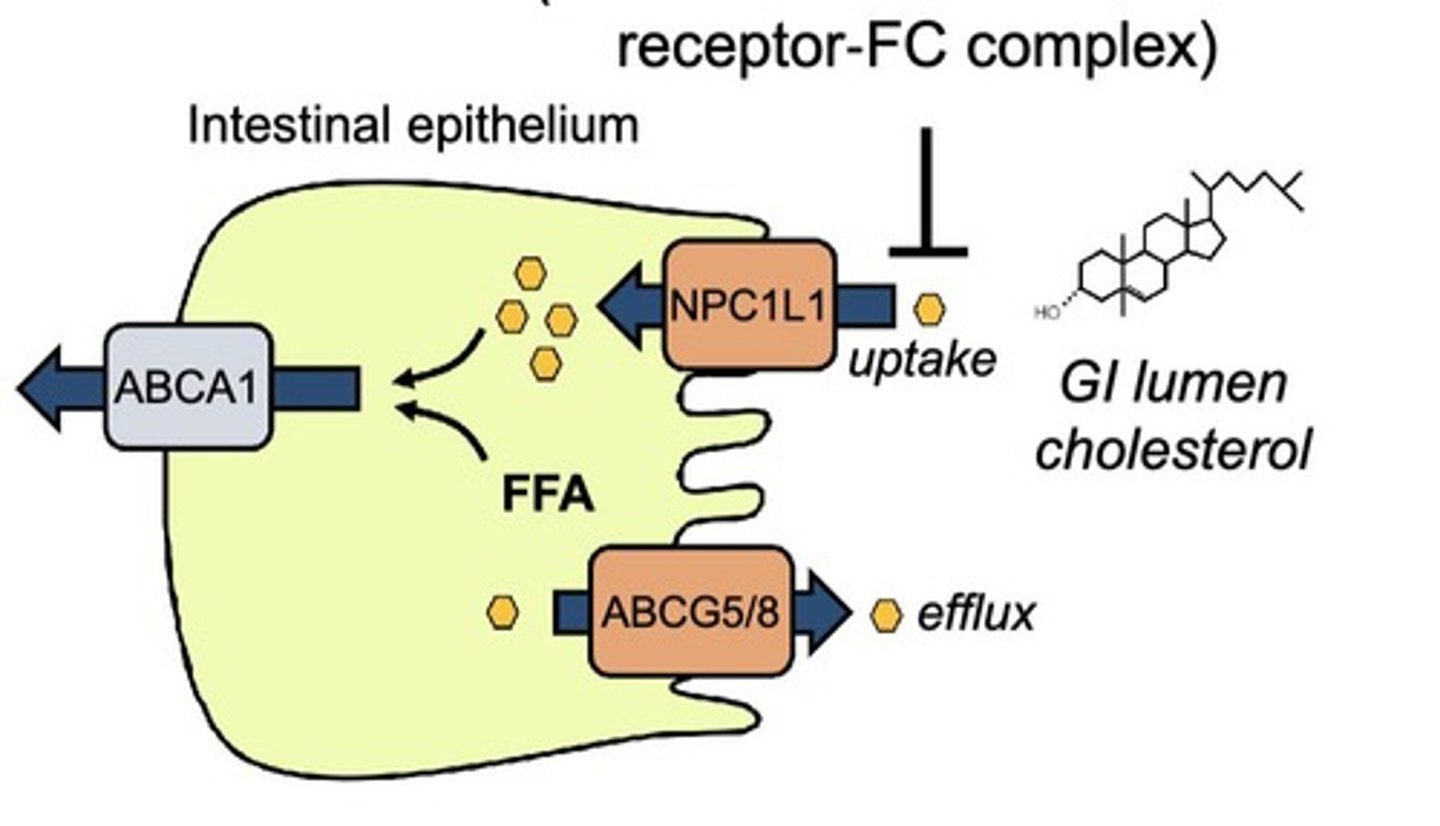
What are the drug interactions of Ezetimibe?
Bile acid sequestrants reduce the absorption of Ezetimibe → it should be administered at least 2 hours before or 4 hours after these agents.
What is the mechanism of Bempedoic acid?
inhibits ATP-Citrate Lyase, blocking the conversion of citrate to acetyl-CoA.
What are the effects of Bempedoic acid?
decreases LDL-C by 17% when used alone
38% when combined with Ezetimibe.
What is the mechanism of bile acid sequestrants?
They bind to negatively charged bile acids in the intestine, preventing their reabsorption and promoting LDL uptake by the liver.
What are the adverse effects of bile acid sequestrants?
increased TG levels
constipation
malabsorption of fat-soluble vitamins.
What is the mechanism of fibric acids?
Fibric acids activate PPARα → increasing lipid metabolism and triglyceride lipolysis.
What are the effects of fibric acids?
decrease triglycerides by 20-50%
may modestly decrease LDL-C
What are the adverse effects of Niacin?
Flushing
pruritis
GI upset
increased uric acid levels
liver toxicity
increased blood sugar.
What are the effects of Omega-3 fatty acids for hyperlipidemia?
They help lower triglyceride levels and may improve overall lipid profiles.
What is the difference between systolic heart failure (HFrEF) and diastolic heart failure (HFpEF)?
HFrEF is characterized by decreased contractility and reduced ejection fraction
HFpEF involves stiff ventricles with preserved ejection fraction.
What are common risk factors for diastolic heart failure?
Age
hypertension (HTN)
diabetes mellitus (DM).
What leads to pulmonary edema in heart failure?
Left ventricular (LV) failure.
What leads to systemic or peripheral edema in heart failure?
Right ventricular (RV) failure.
What factors determine cardiac output (CO)?
Heart rate (HR)
preload
contractility
afterload.
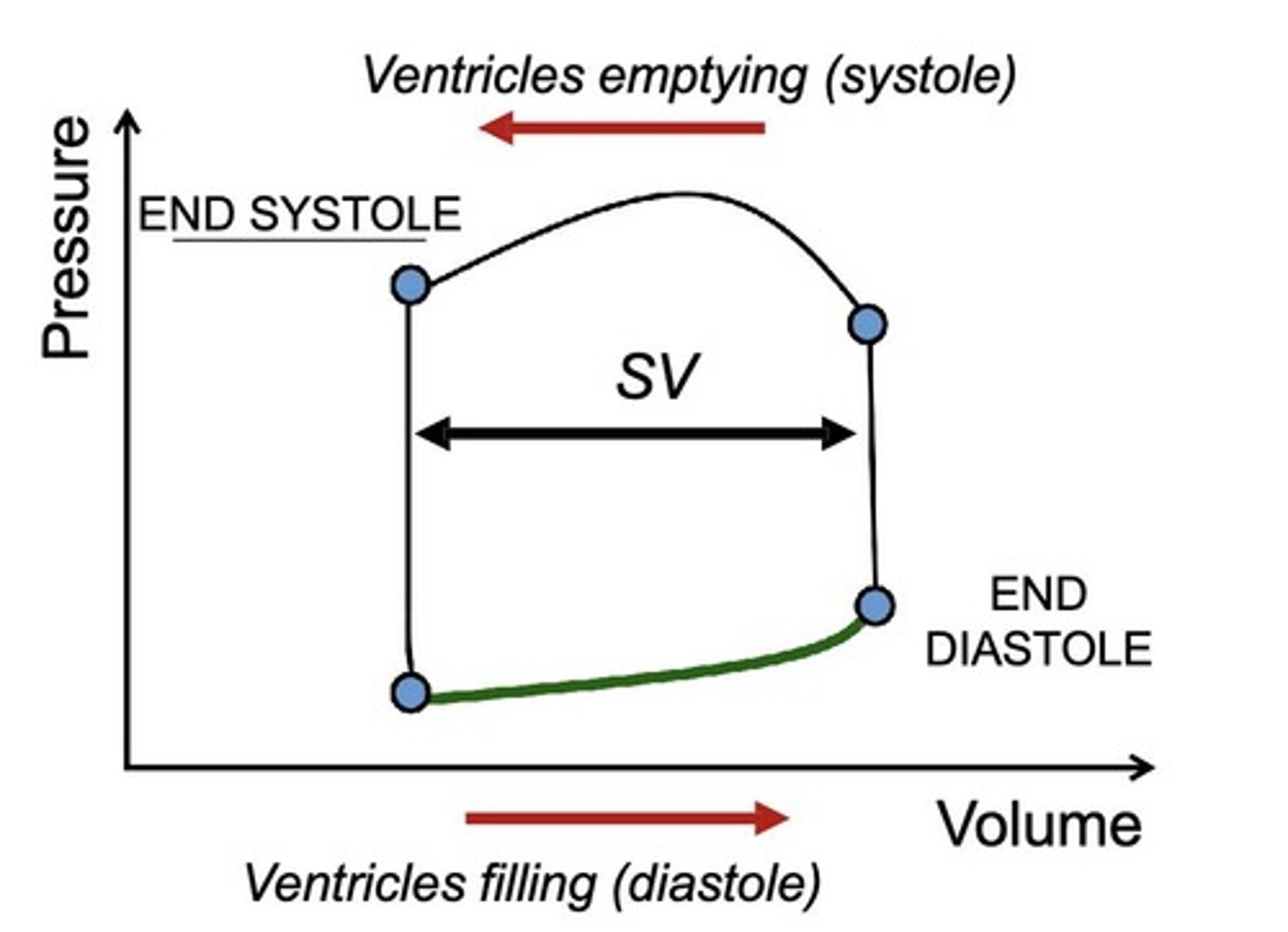
How does preload affect stroke volume (SV)?
increased preload → increases SV, according to the Frank-Starling law.
What is the relationship between preload and central venous pressure (CVP) for the right ventricle?
Preload is proportional to CVP.
What happens to stroke volume in diastolic heart failure?
Stroke volume decreases due to stiffer ventricles, even with normal end-diastolic pressure (EDP).
What is contractility and how is it affected in systolic heart failure?
Contractility is the force generated by the ventricles during systole, which is decreased in systolic heart failure.
What is afterload and how is it influenced?
Afterload is the peak systolic stress that must be overcome for blood ejection, influenced by systolic pressure and ventricular radius.
How does high systolic blood pressure affect afterload?
High systolic blood pressure increases afterload.
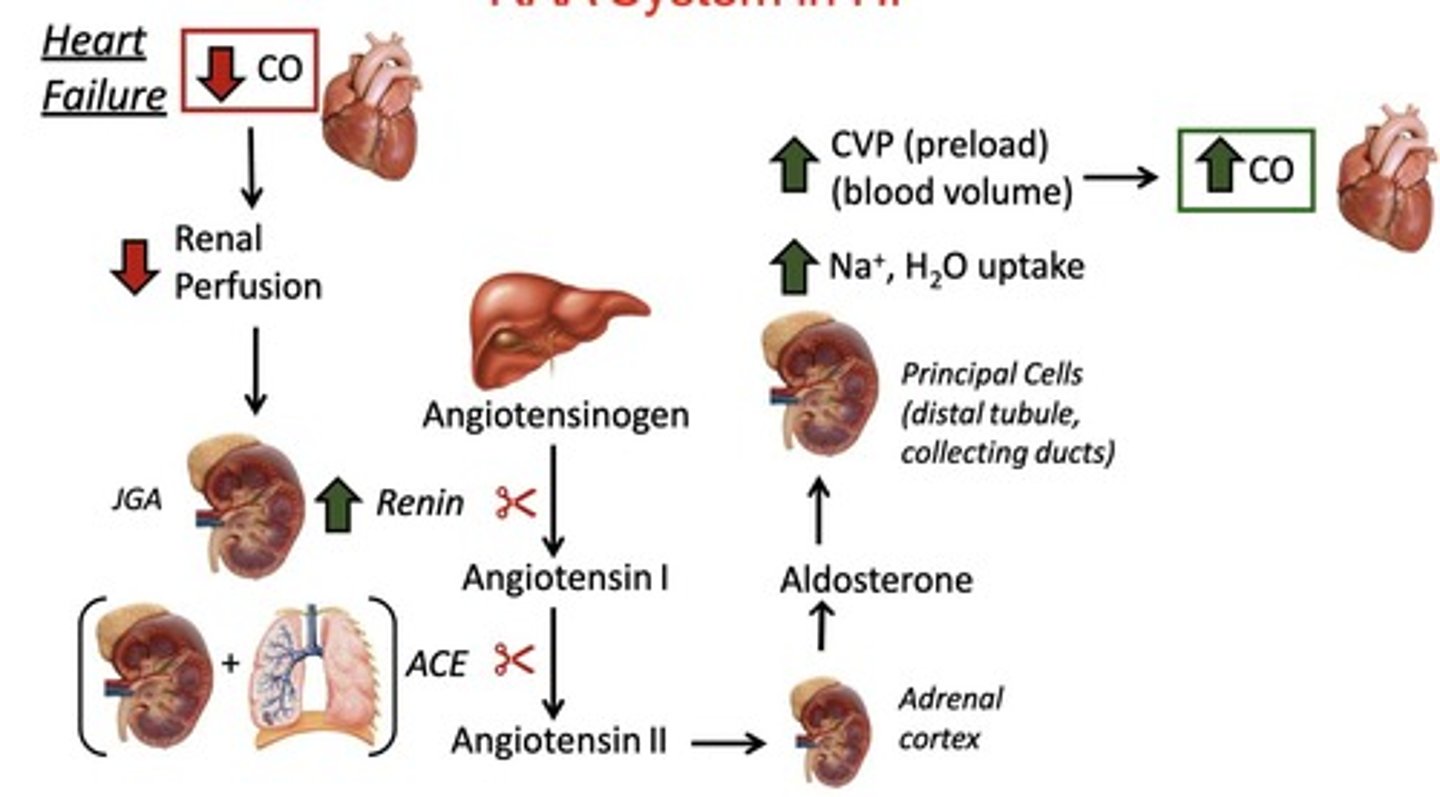
What role does the RAAS system play in heart failure?
RAAS activation leads to:
decreased cardiac output
renal perfusion
increased renin
angiotensin II-induced vasoconstriction
aldosterone-mediated sodium and water retention.
What are the effects of sympathetic activation in heart failure?
increased heart rate
increased contractility
vasoconstriction
side-note: long-term can lead to cardiac remodeling and worsening heart failure.
What are the main electrophysiological processes targeted by antiarrhythmics?
Automaticity
conduction velocity
refractoriness.
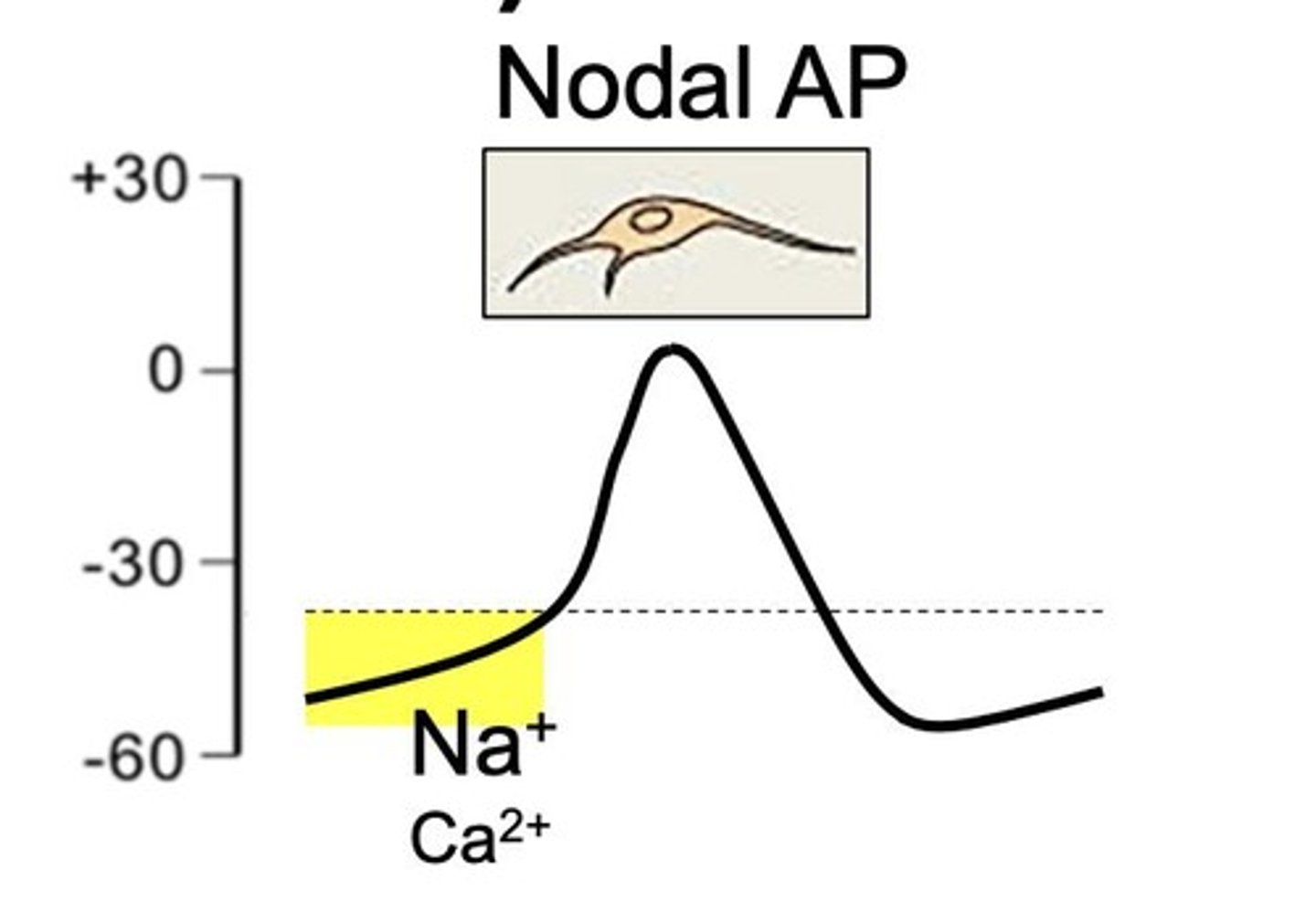
What is automaticity in cardiac tissues?
Automaticity is spontaneous depolarization in nodal tissues due to funny current (If) and occurs in phase 4.
What is the significance of ectopic foci in arrhythmias?
Ectopic foci can form in diseased myocytes, leading to abnormal pacemaker activity and interference with normal sinus rhythm.
How does conduction velocity relate to cardiac depolarization?
Conduction velocity depends on the slope of phase 0 depolarization, with nodal tissues primarily relying on calcium influx.
What is refractoriness and how is it affected by K+ channel blockade?
Refractoriness is the period during which depolarization is not possible; K+ channel blockade prolongs this period, increasing effective refractory period (ERP).
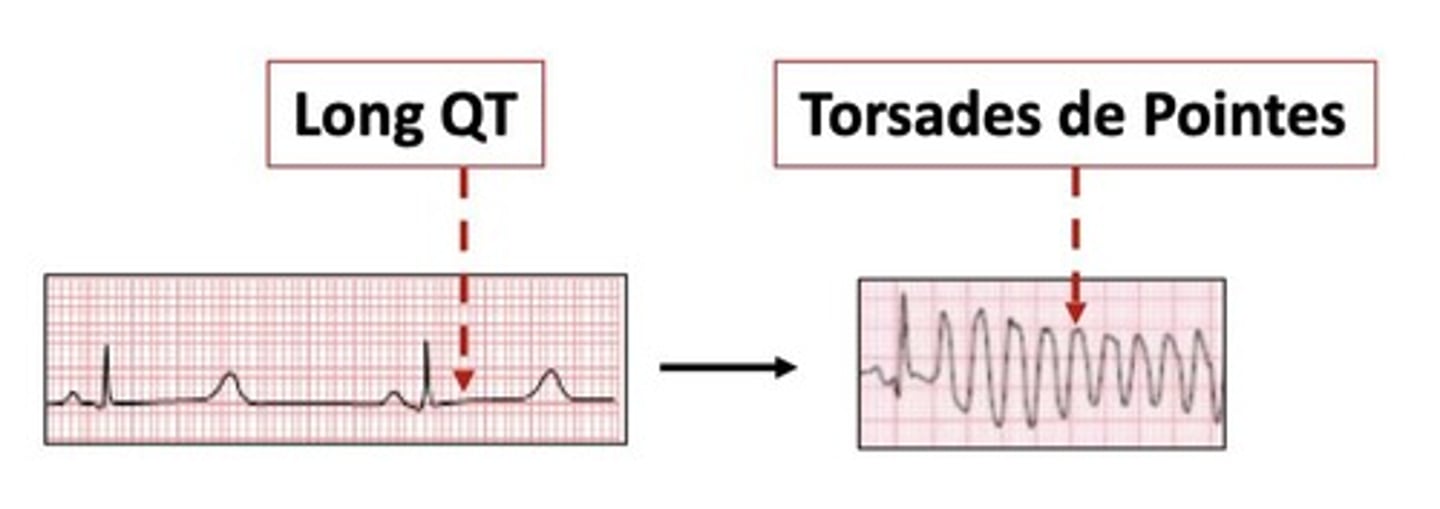
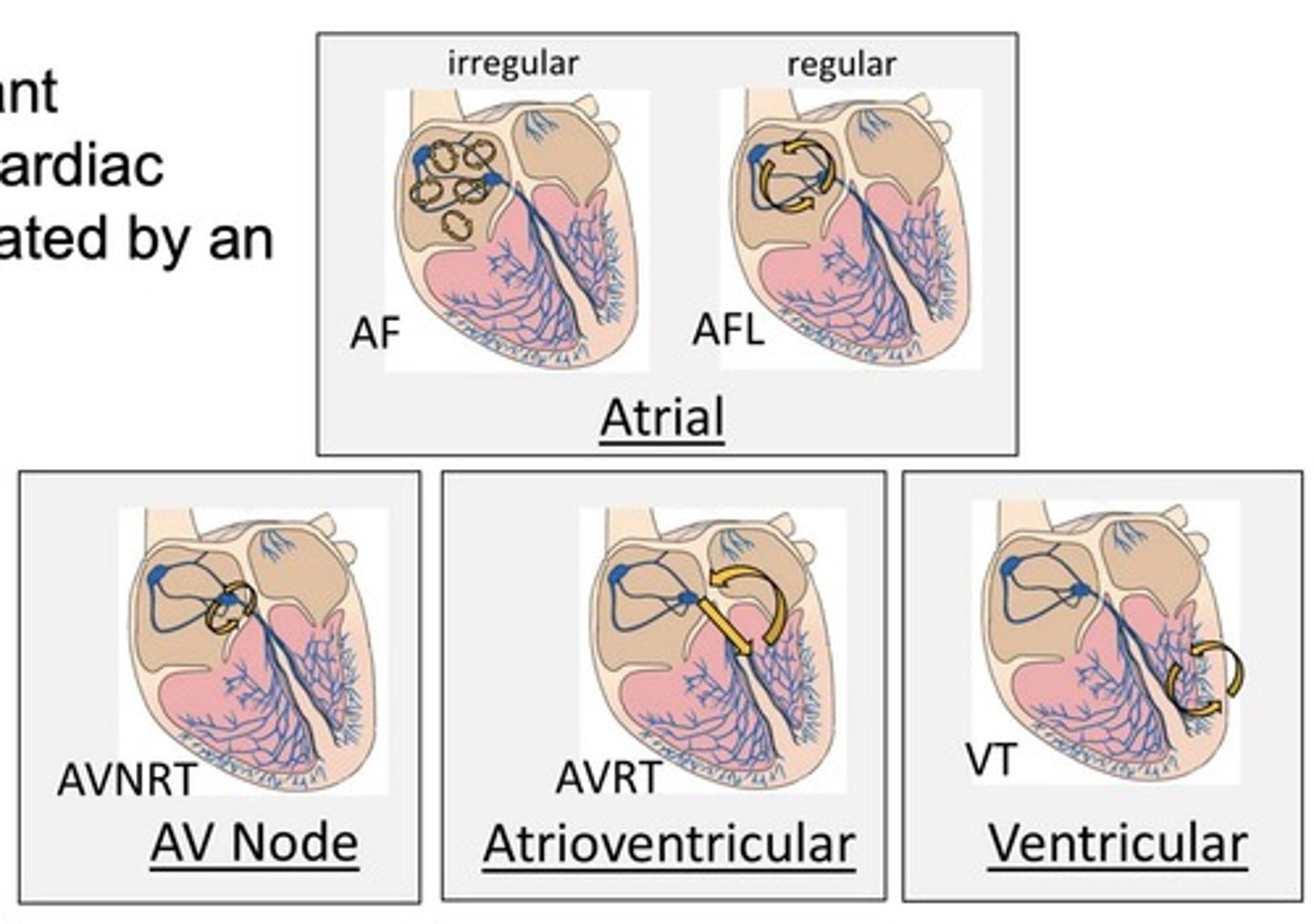
How can arrhythmias be classified by location?
Arrhythmias can be classified as supraventricular (SA, AV, atrial), ventricular, or junctional.
What is the mechanism of enhanced automaticity in arrhythmias?
Enhanced automaticity involves accelerated generation of action potentials by normal pacemaker cells due to increased depolarization.
What are examples of ectopic rhythms?
atrial premature beats
atrial tachycardias
multifocal atrial tachycardia
ventricular tachycardia.
What is reentry in the context of arrhythmias?
Reentry is when impulses die out and reentrant impulses circle back to re-excite cardiac regions other than the SA node.
What are examples of reentrant arrhythmias?
atrial fibrillation (AF)
atrial flutter (AFL)
AV node reentry (AVNRT)
AV reentry (AVRT)
ventricular tachycardia (VT).
How do drugs affect reentry in arrhythmias?
Drugs can suppress reentry by:
increasing ERP (Class III, Ia)
decreasing conduction velocity (Class Ic, II, IV).
What is the difference between rate control and rhythm control in atrial fibrillation?
rate control slows AV conduction (e.g., β-blockers)
rhythm control aims to restore sinus rhythm (e.g., Na+ and K+ channel blockers).
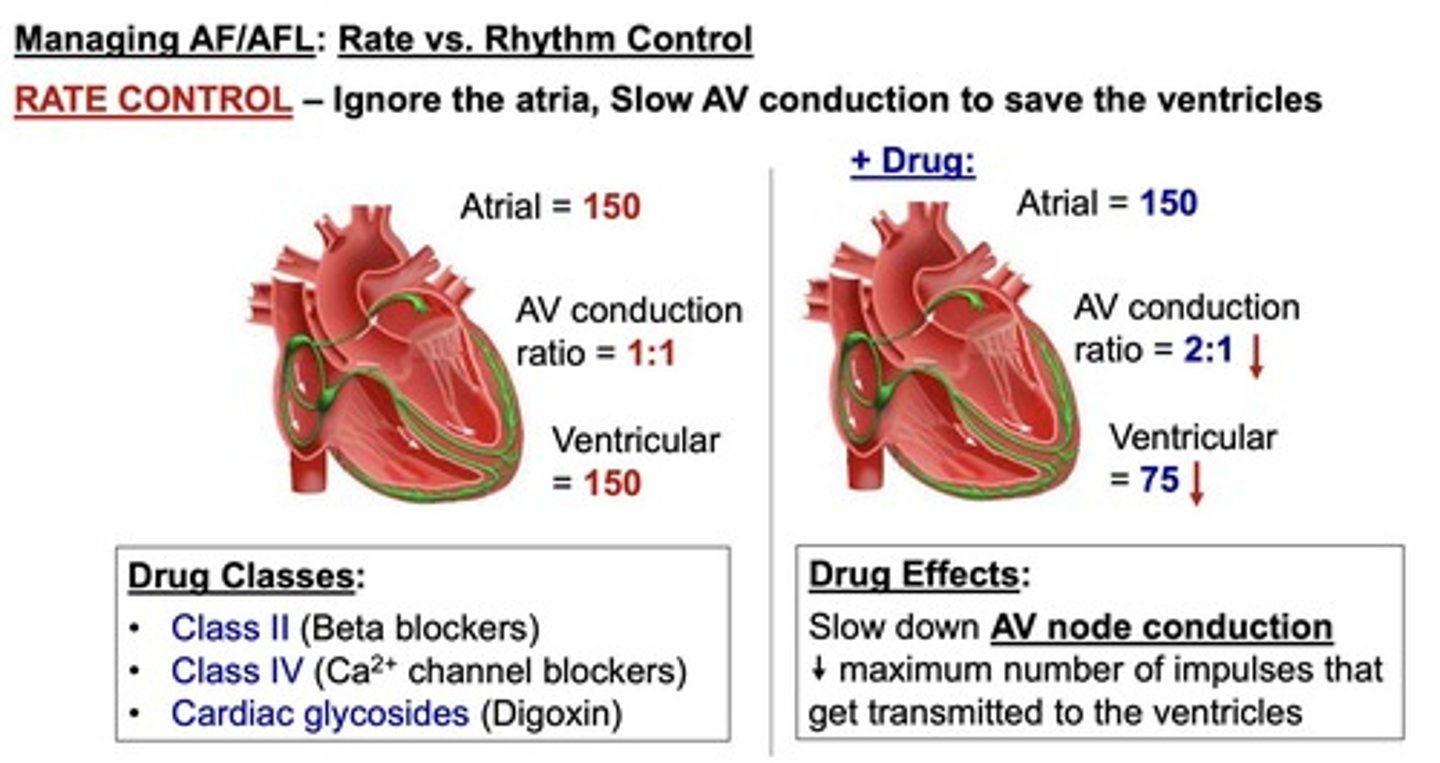
What is the mechanism of loop diuretics in heart failure?
Loop diuretics inhibit Na+/K+/2Cl-reabsorption in the loop of Henle, acting as the strongest diuretics.
What is a potential consequence of excessive diuresis in heart failure?
can worsen cardiac output and activate the RAAS system, leading to vasoconstriction and fluid retention.
What is the mechanism of action of loop diuretics?
They inhibit the Na/K/Cl symporter in the loop of Henle, affecting Ca and Mg levels.
What is the site of action for loop diuretics?
Loop of Henle (LOH).
What are common adverse effects of loop diuretics?
hypokalemia/-calcemia/-magnesemia
hyperlipidemia
ototoxicity
sulfa allergy (except ethacrynic acid).
What drug interactions are associated with loop diuretics?
NSAIDs → decrease GFR
ACE inhibitors/ARBs → decrease GFR
corticosteroids → additive hypokalemia
What is the mechanism of action of thiazide diuretics?
They block the Na/Cl cotransporter in the distal convoluted tubule (DCT), enhancing Ca²⁺ reabsorption.
What are examples of thiazide diuretics?
Chlorothiazide
Chlorthalidone
Metolazone.
What is the mechanism of action of potassium-sparing diuretics?
They block aldosterone receptors (Spironolactone, Eplerenone) or inhibit ENaC channels (Amiloride, Triamterene).
What are the adverse effects of Spironolactone?
gynecomastia
sexual dysfunction.
What is the mechanism of action of ACE inhibitors?
They block the conversion of Angiotensin I to Angiotensin II, increasing bradykinin levels.
What are common adverse effects of ACE inhibitors?
dry cough
angioedema
hyperkalemia
hypotension
renal dysfunction.
What distinguishes ARBs from ACE inhibitors?
ARBs block AT1 receptors, preventing Ang II from binding, and have less bradykinin effect.
What are the adverse effects of ARBs?
Hypotension
hyperkalemia
renal dysfunction
no cough or angioedema.
What is the mechanism of action of beta-blockers?
They block beta-adrenergic receptors, decreasing heart rate and contractility.
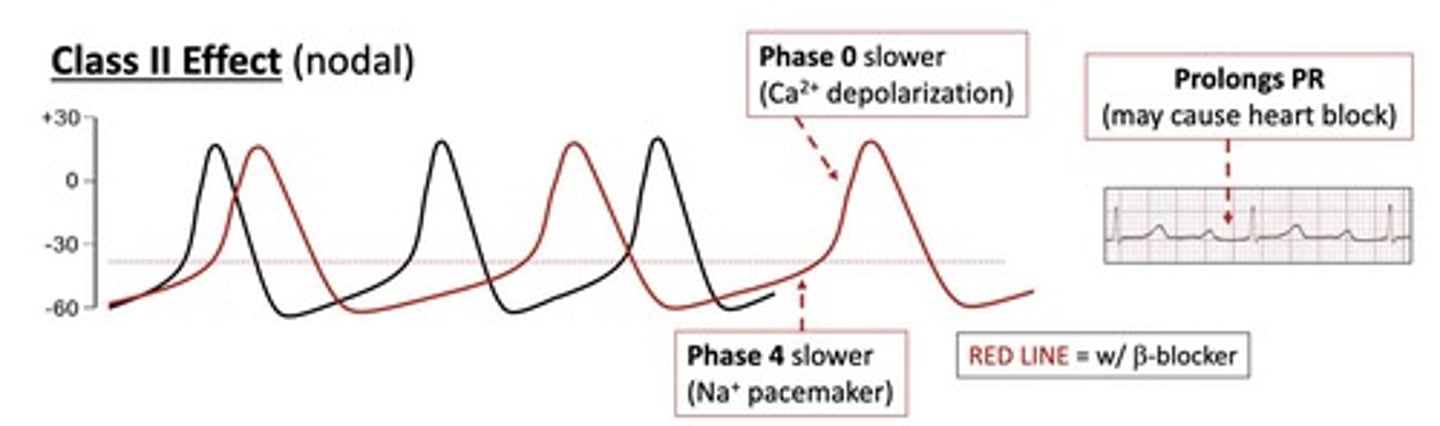
What are common adverse effects of beta-blockers?
bradycardia
fatigue
dizziness
withdrawal syndrome.
What is the mechanism of action of digoxin?
It inhibits Na⁺/K⁺ ATPase, increasing intracellular Ca²⁺ and contractility.
What is the therapeutic window for digoxin?
Narrow (0.5-1.0 ng/ml), with toxicity occurring >2 ng/ml.
What are the adverse effects of digoxin toxicity?
NVD
arrhythmias
CNS confusion
yellow vision (xanthopsia).
What is the mechanism of action of PDE3 inhibitors like Milrinone?
They increase cAMP levels, leading to increased intracellular Ca²⁺ and vasodilation.
What are the adverse effects of PDE3 inhibitors?
headaches
hypotension
ventricular arrhythmias
chest pain.
What are the five classes of antiarrhythmic drugs according to the Vaughan-Williams classification?
Class I - Na Channel Blockers
Class II - B-Blockers
Class III - K Channel Blockers
Class IV - Ca Channel Blockers,
Class V - Other.
What is the mechanism of Class Ia antiarrhythmics?
They slow depolarization, prolong QRS and QT intervals, and may have anticholinergic effects.