Kaplan MCAT General Chemistry Chapter 11: Oxidation-Reduction Reactions
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Oxidation
Loss of electrons
Reduction
Gain of electrons
Oxidation-reduction reactions
any chemical change in which one species is oxidized (loses electrons) and another species is reduced (gains electrons); also called redox reaction
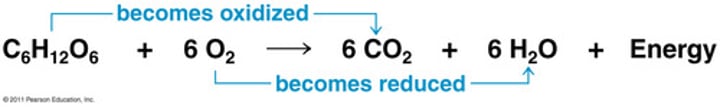
Oxidizing agent
Facilitates the oxidation of another compound and is REDUCED itself in the process.
Reducing agent
Facilitates the reduction of another compound and is itself OXIDIZED in the process.
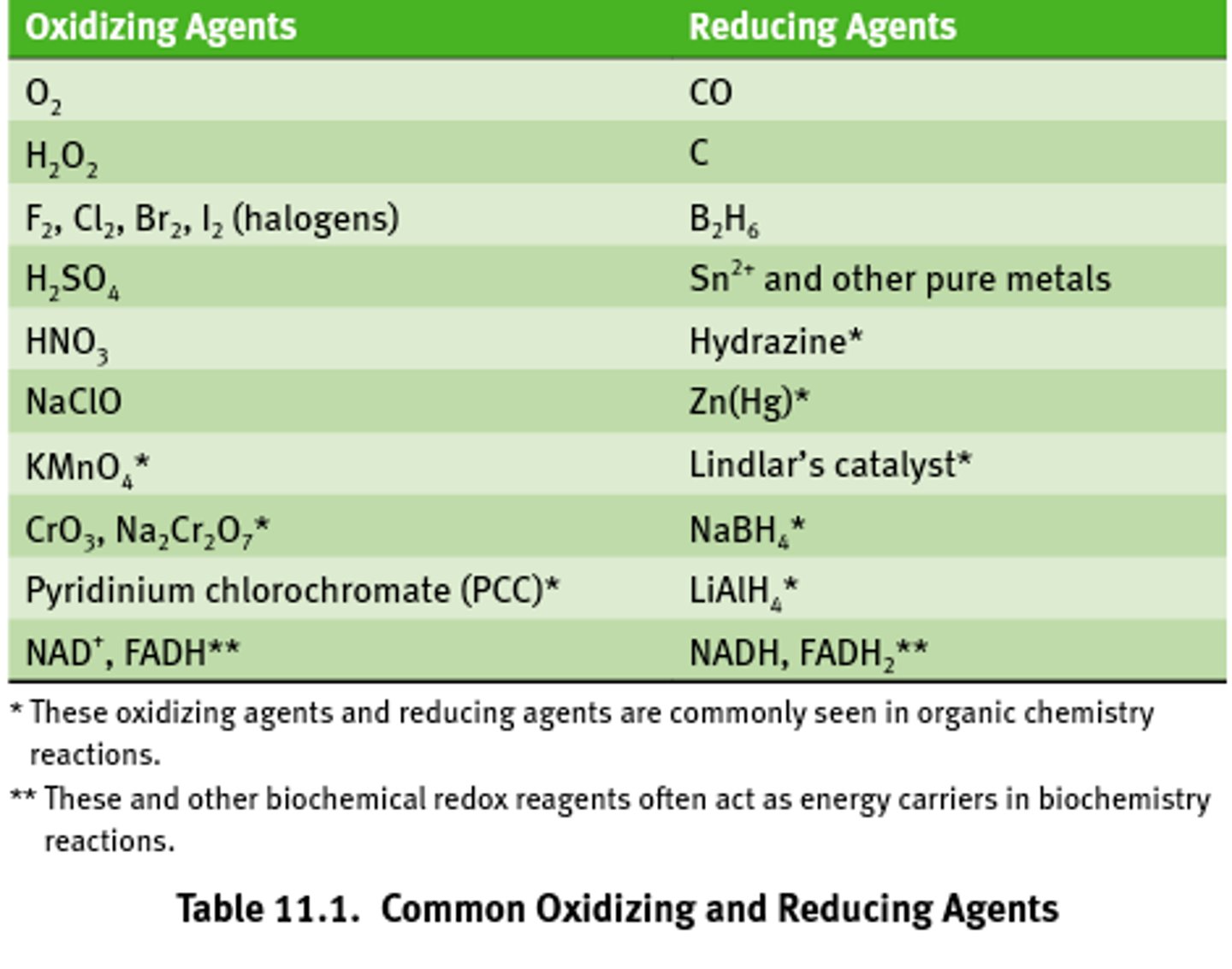
Common oxidizing agents
Almost all oxidizing agents contain oxygen and similarly electronegative elects.
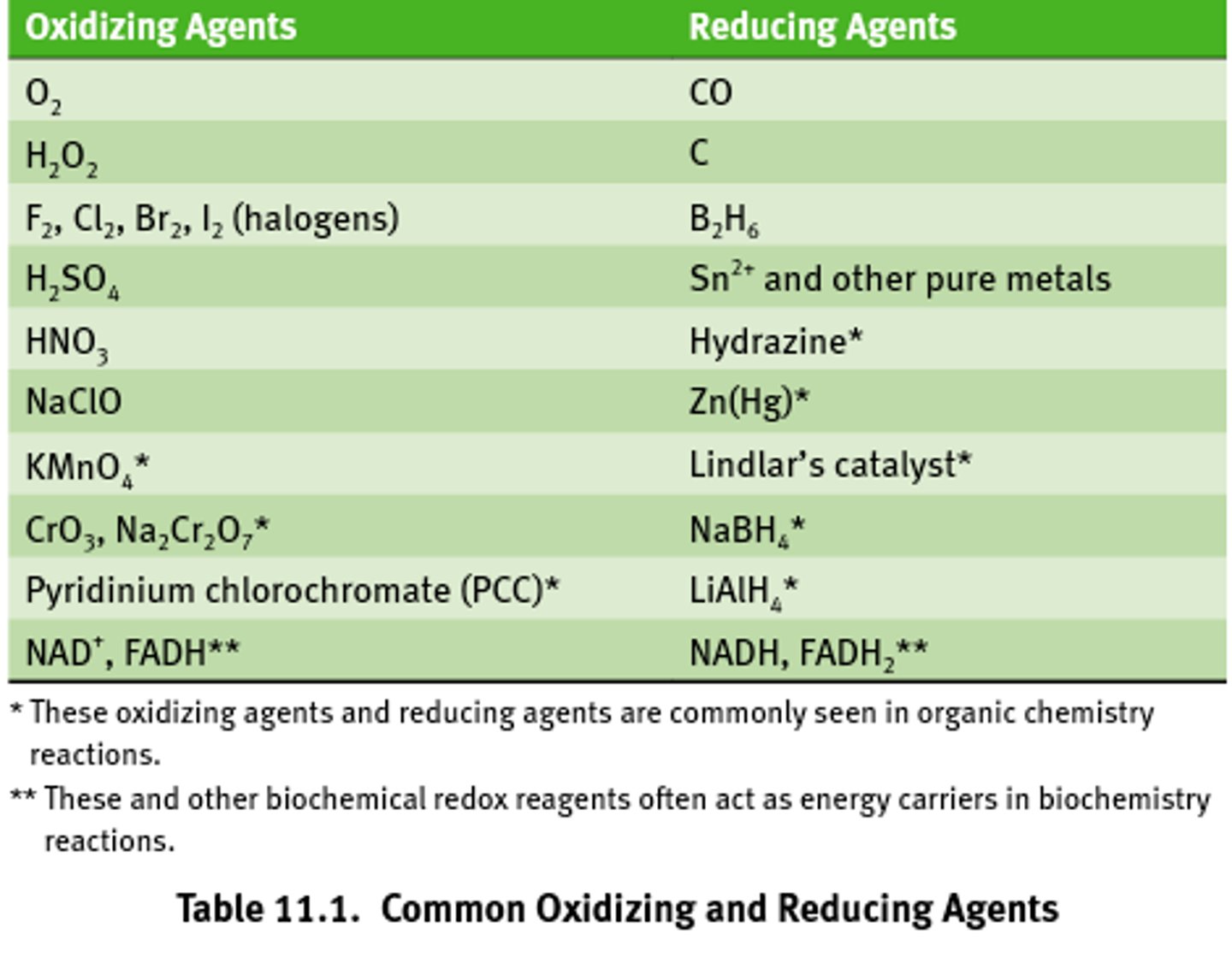
Common reducing agents
Often contain metal ions and hydride ions (H-)
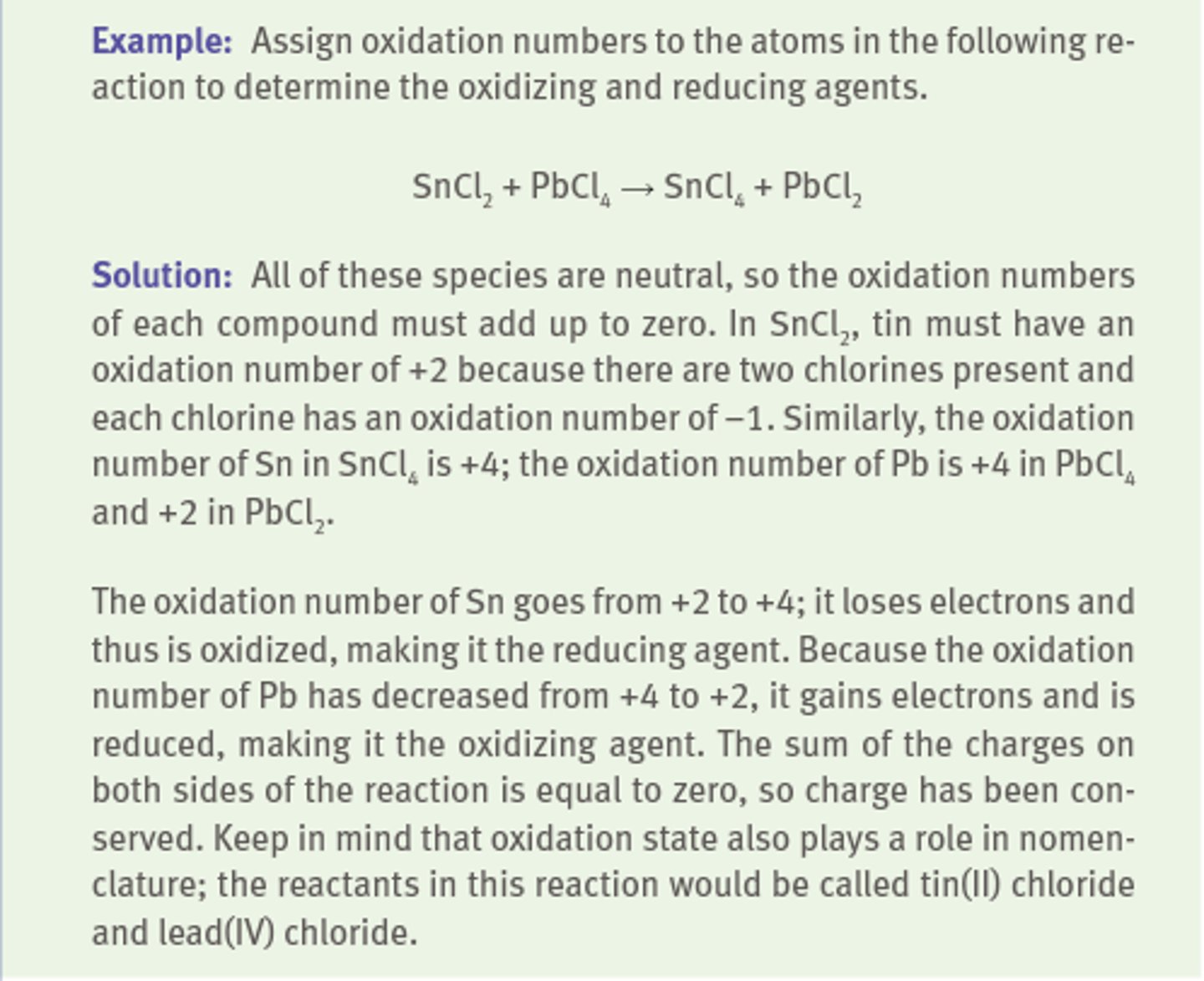
Assigning oxidation numbers
(RULES)
To assign oxidation numbers correctly, one must know the common oxidation states of the representative elements:
1) Free atoms or diatomic species have an oxidation number of zero. (Ex. N2, P4, S8 all have an oxidation number of zero)
2) The oxidation number of monoatomic ions is equal to the charge of the ion. (ex: Na+ oxidation number is +1)
3) When in compounds, Group IA metals have an oxidation number of +1, Group IIA have oxidation number of +2.
4) When in compounds, Group VIIA elements have an oxidation number of -1 (unless combined with an element with higher electronegativity) (ex: in HCl, Cl oxidation number is -1, in HOCl its oxidation number is +1)
5) The oxidation number of hydrogen is +1 unless it is paired with a less electronegative element, in which case it is usually -1. (ex: in HCl, H oxidation number is +1, in NaH its oxidation number is -1)
6) The oxidation number of oxygen is usually -2, except in peroxides (when its charge is -1), or in compounds with more electronegative elements. (ex: in OF2, O's oxidation number is +2)
7) The sum of the oxidation numbers of all compounds present in a compound must equal the overall change of the compound. (ex: for SO4^-2, the sum of the oxidation numbers must be -2)
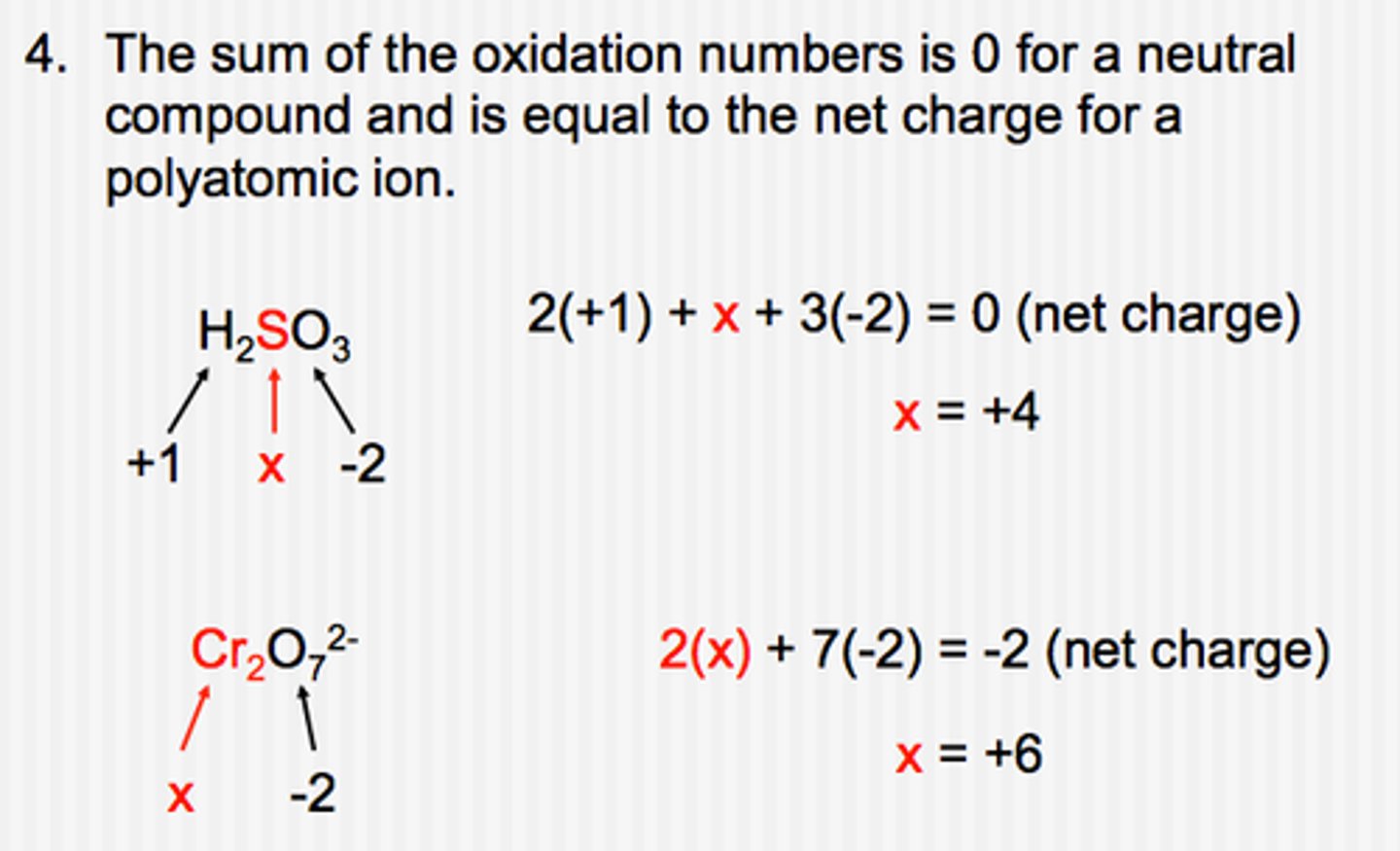
Problem example
Half-reaction method
A method used to balance redox reactions:
1) Separate the two half-reactions
2) Balance the atoms of each half reaction. Start with all the elements besides H and O, balance H and O using water and H+. In basic solution, balance H and O using water and OH-.
3) Balance the charges of each half-reaction by adding electrons as necessary to one side of the reaction.
4) Multiply the half-reactions as necessary to obtain the same number of electrons in both half-reactions.
5) Add the half-reactions, canceling out terms on both sides of the reaction arrow.
6) Confirm that the mass and charge are balanced.
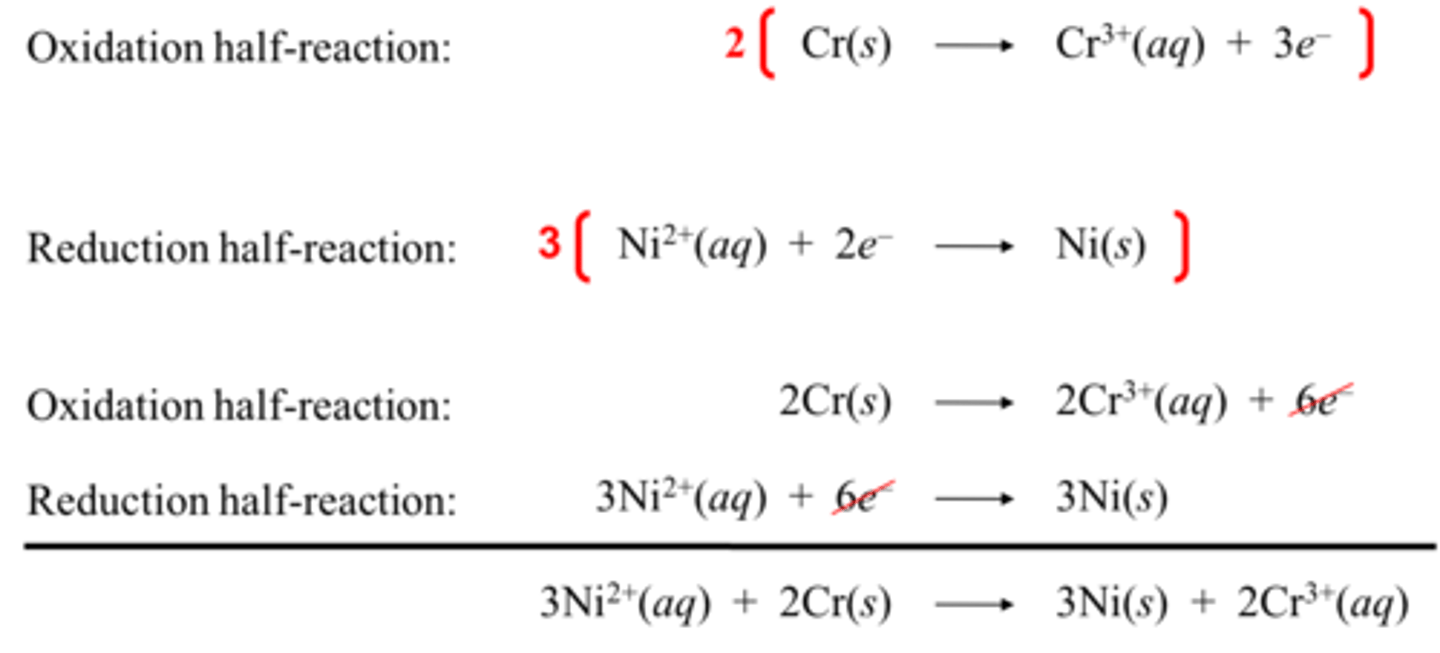
Problem Example half-reaction

Complete ionic equation
Accounts for all of the ions present in the reaction. To write the complete ionic reaction, split all aqueous compounds into their relevant ions. Keep solid salts intact.
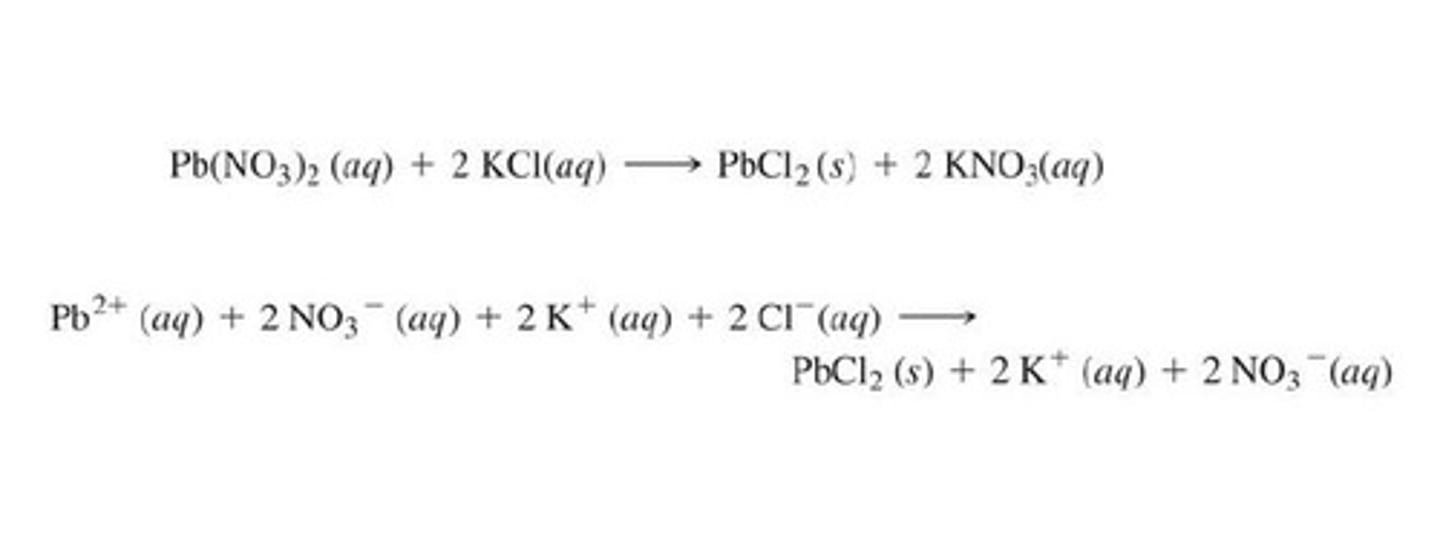
spectator ions
Ions that do not take part in a chemical reaction and are found in solution both before and after the reaction
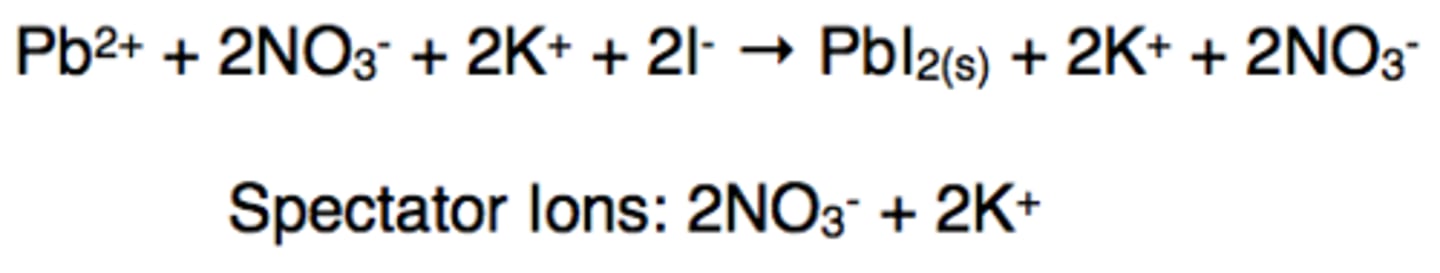
Net ionic equation
Ignore spectator ions to focus only on the species that actually participate in the reaction. To obtain a net ionic reaction, subtract the ions appearing on both sides of the reaction (spectator ions).
1) for reactions with no aqueous salts, the net ionic equation is generally the same as the overall balanced equation.
2) For double displacement reactions that do not form a solid salt, there is no net ionic reaction because all the ions remain in solution and do not change the oxidation number.
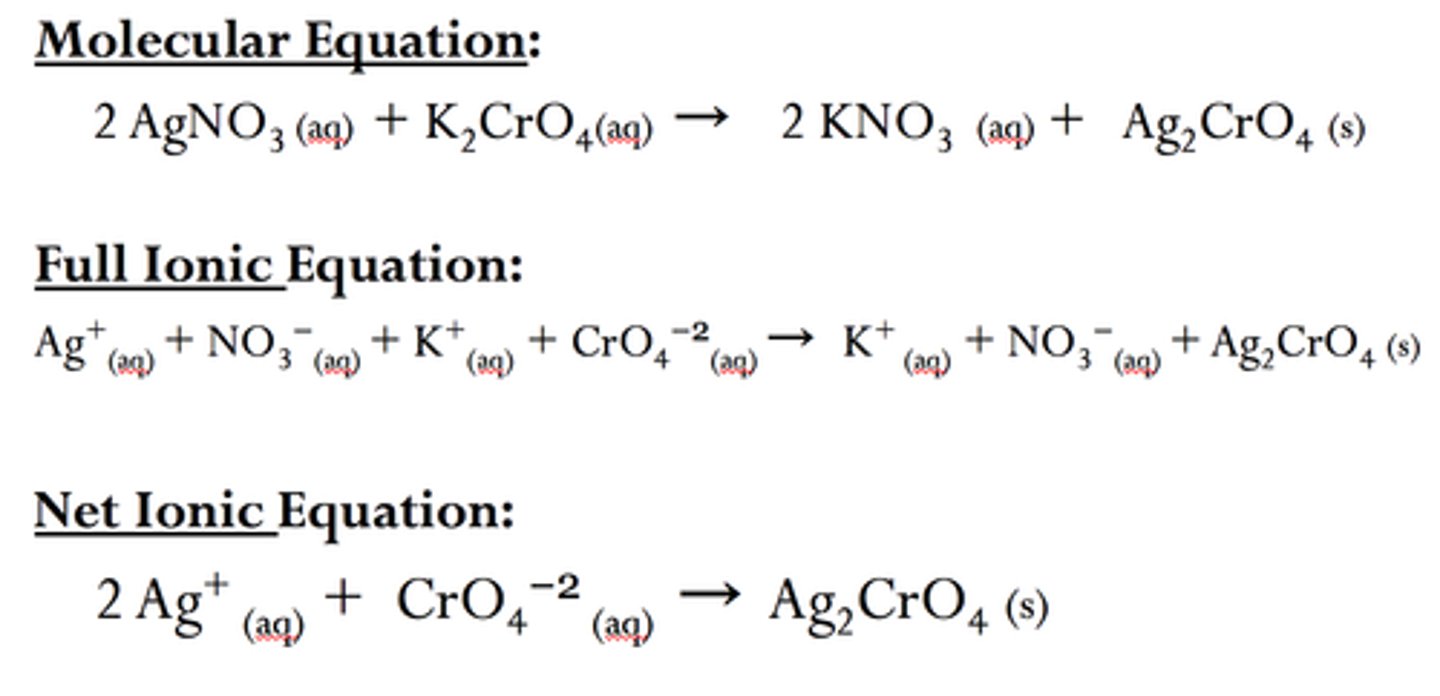
combination reaction
a chemical change in which two or more substances react to form a single new substance
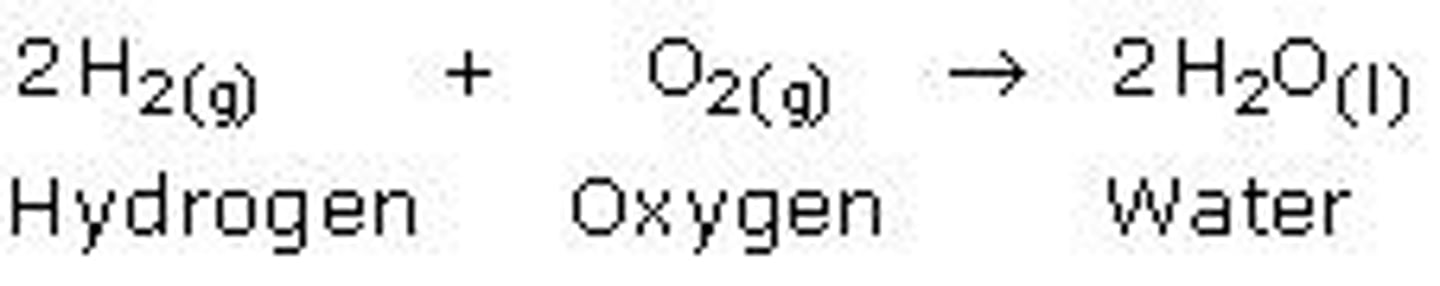
decomposition reaction
a reaction in which a single compound breaks down to form two or more simpler substances
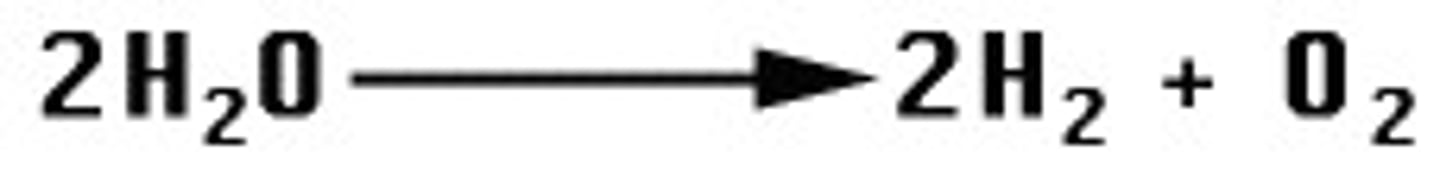
combustion reaction
a reaction where a fuel (usually a hydrocarbon) is mixed with an oxidant (usually oxygen) to from carbon dioxide and water
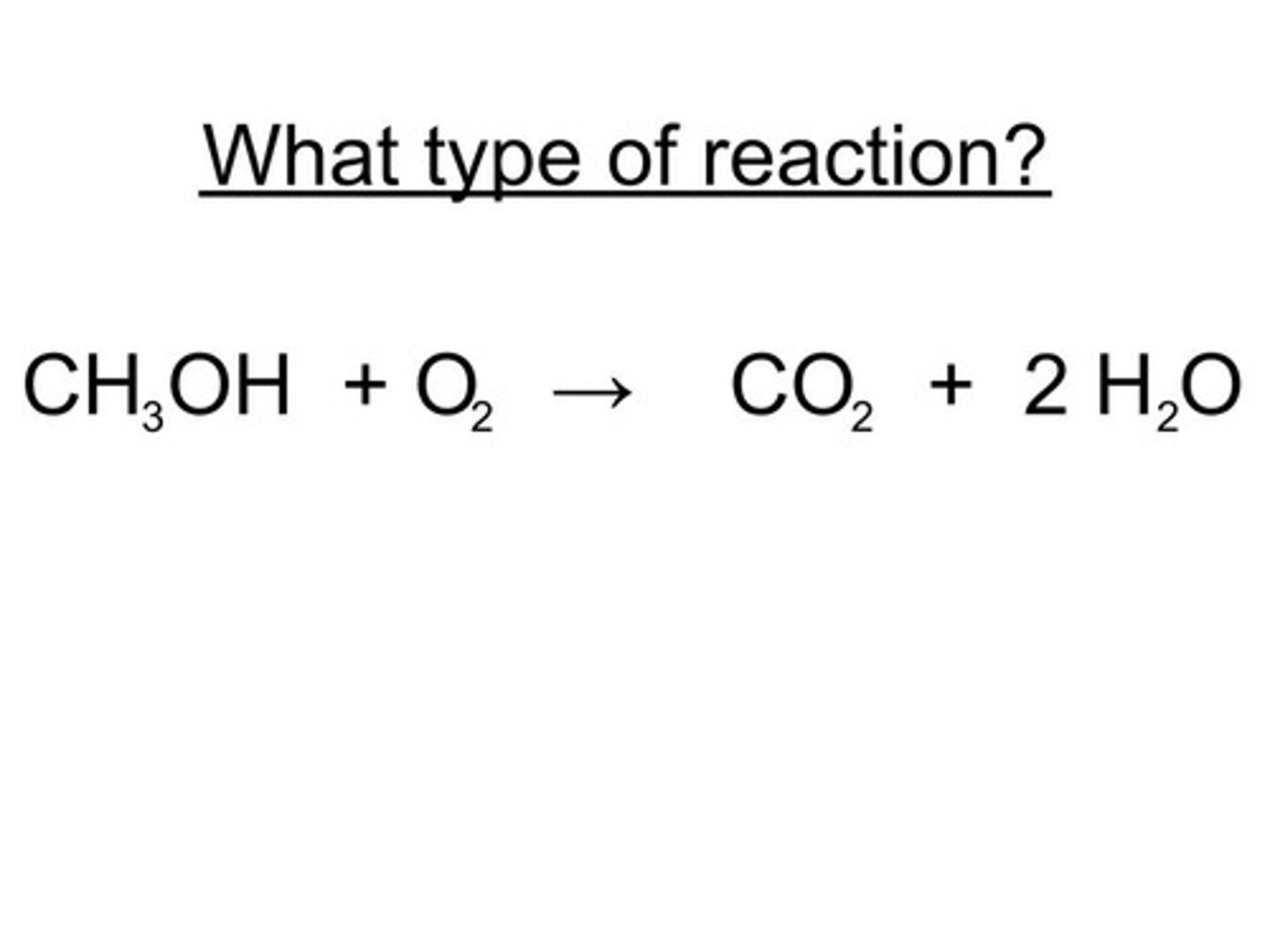
double displacement reaction
a reaction that involves the witching of counter-ions, because all ions retain their oxidation state, these are not oxidation reduction reactions
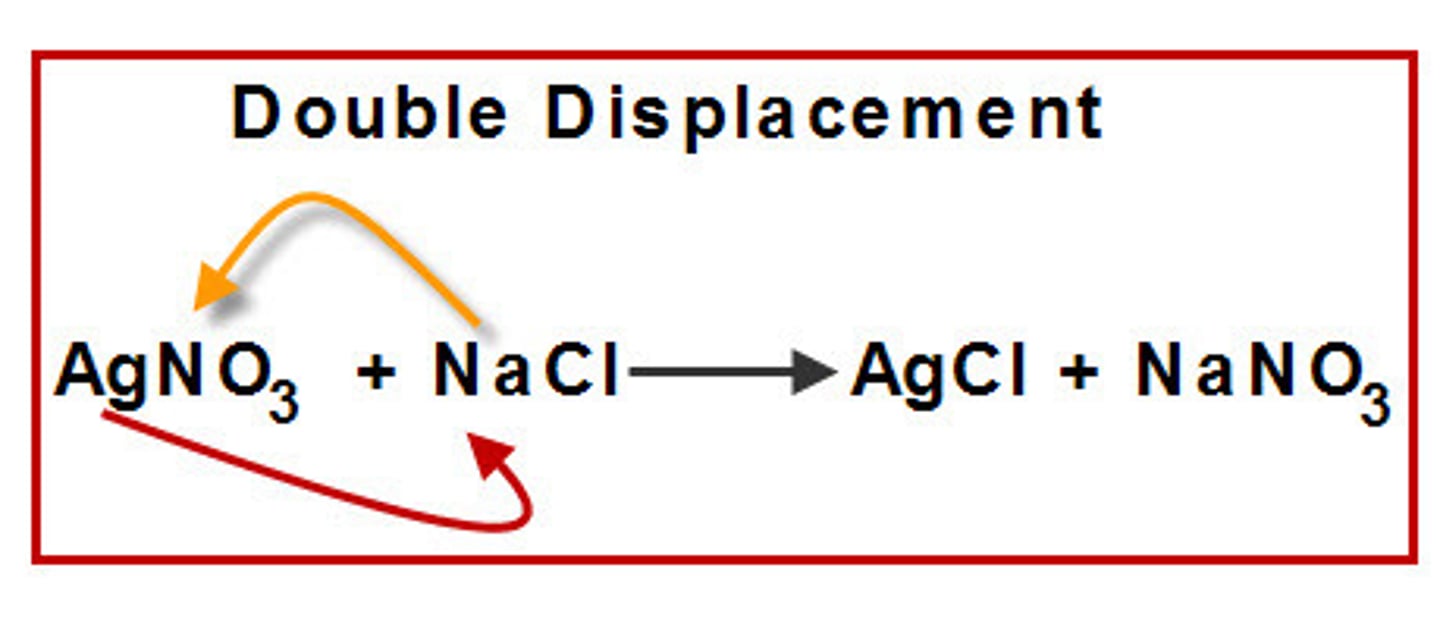
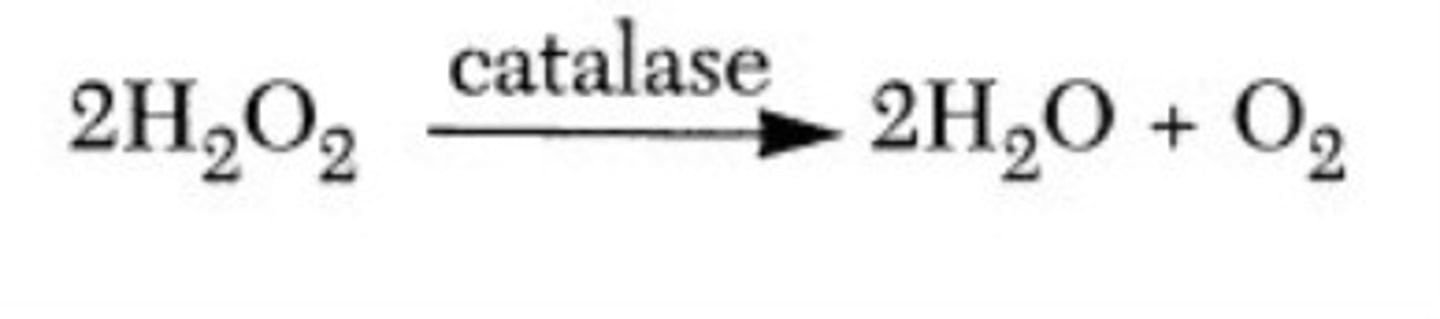
Disproportionation reactions
Are a type of redox reaction in which one element is both oxidized and reduced, forming at least two molecules containing the element in different oxidation states.
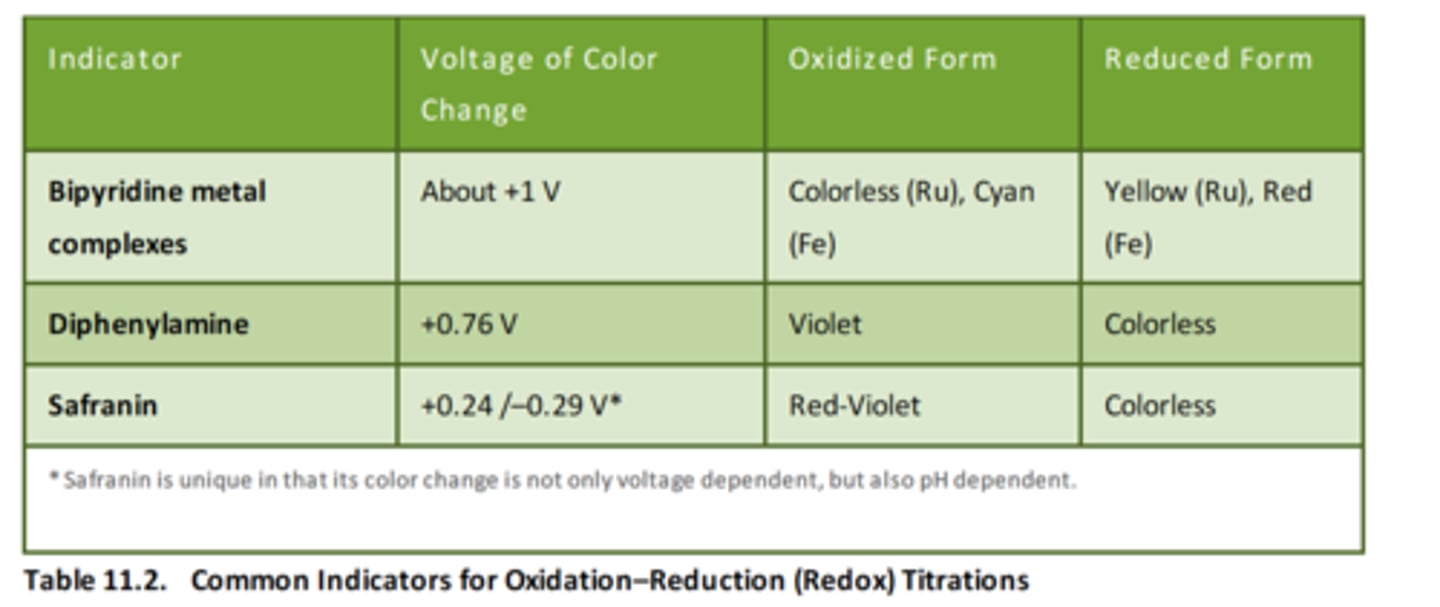
Oxidation-reduction titrations
Similar in methodology to acid-base titrations. These titrations follow the transfer of charge.
Potentiometric titrations
A form of redox titration in which the voltmeter or external cell measures the electromotive force of the solution. No indication is used, and the equivalence point is determined by a sharp change in voltage.