Rates of Reaction and Energy Changes
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
How does increasing concentration affect the rate of reaction?
If there are more particles then there will be a higher number of successful collisions
How does increasing temperature affect the rate of reaction?
The particles gain energy, making them move faster, leading to a higher amount of collisions quickly
The particles hit harder so the collision has a higher chance of being successful
How does increasing surface area affect the rate of reaction?
If something has a larger surface area, there are more places for particles to collide with, leading to more successful collisions
What is the meaning of the rate of reaction?
The speed at which reactants are turned into products
What term should we use when talking about chemical reactions making products?
Successful collisions
Why will a reaction slow down near the end?
Because there are less reactants than what we started with as they were all used up
What do we call a substance that can speed up the rate of reaction?
A catalyst
Why are catalysts useful?
They reduce activation energy so high temperatures aren’t needed
They are never used up which saves money
They speed up the rate of reaction
They don’t alter the products of a reaction
If we know that increasing surface area increases the rate of reaction, why would a powder react faster than a larger chunk of a substance?
Because the powder is grinded up into smaller pieces, there is a larger overall surface area so there are more surfaces for successful collisions to occur
What is an exothermic reaction and what will happen to bond in an exothermic reaction?
A reaction that gives off heat
Energy is transferred to the surroundings as bonds form, so making bonds is exothermic
What is an endothermic reaction and what happens to bonds in one?
A reaction which takes in heat
Energy is transferred to the reactants to break their bonds, so breaking bonds in endothermic
What happens to the temperature of surroundings in an exothermic reaction?
Temperature of surroundings increases
What happens to the temperature of surroundings in an endothermic reaction?
Temperature of surroundings decreases
How do we calculate bond energy?
Calculate energy in (Bonds broken)
Calculate energy out (Bonds made)
Energy Change=energy in - energy out
A negative answer=exothermic and a positive answer=endothermic
What are the requirements for a reaction to happen?
The reactant particles must collide with each other
The collisions must have enough energy
What is activation energy?
The minimum energy needed by reactant particles for a reaction to happen
How does increasing pressure increase the rate of reaction?
There are more particles in the same volume
The frequency of successful collisions increases
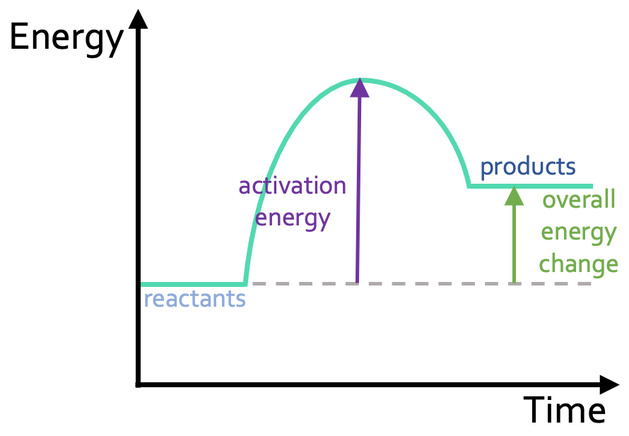
What should we see in an exothermic reaction profile?
The energy level of the reactants is greater than the energy level of the products
The energy change of the reaction is negative ( energy is transferred to the surroundings)
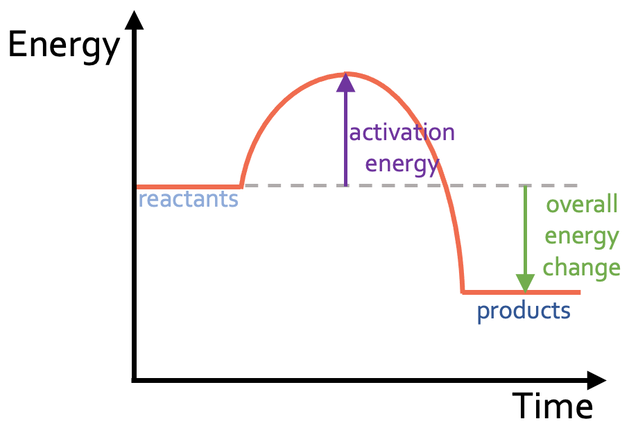
What should we see in an endothermic reaction profile?
The energy level of the reactants is lower than the energy level of the products
The energy change of the reaction is positive (energy is transferred from the surroundings to the products)
How can activation energy be supplied?
Heating the reaction mixture
Applying a flame or spark
Continually Heating the reaction mixture
Passing an electric current through an electrolyte (as in electrolysis)
What is bond energy?
The energy needed to beak 1 mol of a particular covalent bond