Valence Bond Theory [Part I]
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Scientific theory
A strongly supported explanation for observed natural laws or large bodies of experimental data.
Must explain the experimental data and be able to predict behavior
Two things that a scientific theory must do in order to be accepted.
Because it predicts three-dimensional molecular shapes that are consistent with the experimental data collected for thousands of different molecules.
Why has the VSEPR theory gained such a widespread acceptance?
To provide an explanation of chemical bonding.
What does the VSEPR theory fails to do?
Quantum mechanics
The tool that is used to predict where electrons are likely to be located in atoms
Spherical
s orbital’s shape
Dumbbell-shaped
p orbitals shape
free atoms, electron distributions in molecules
Atomic orbitals only describe _________ and not _____________________________. This is why atomic orbitals alone are not enough to describe bonding in molecules.
Valence bond theory
Theory that describes a covalent bond as the overlap of half-filled atomic orbitals that combine to form a shared electron pair.
overlap
Orbitals on two different atoms _______ when a portion of one orbital and a portion of second orbital occupy the same region of the space.
1) Orbitals must overlap 2) Single electrons in each orbital combine into a pair.
The two conditions to be met for a covalent bond to formed, according to the valence bond theory.
Covalent bond
The force that physically links two atoms through the mutual attraction between a shared negatively charged electron pair and both atoms’ positively charged nuclei
The extent of orbital overlap
Determines the strength of a covalent bond
stronger
Orbitals that overlap extensively form bonds that are ________ than those that have less overlap.
Energy of two hydrogen atoms when they are far apart.
No orbital overlap
By convention, the sum of energies is set to zero.
electrons, electrons, nuclei, repel
Each ________ feels attraction to the other nucleus, while ________ and ______ also _____ each other.
Happens when hydrogen atoms move closer and orbitals begin to overlap.
A bond begins to form
Atoms are still widely apart from each other, however the attractions are slightly stronger than repulsions, so the system’s energy drops
Nuclear-electron attractions increase, electron-electron/nucleus-nuclear repulsions increase.
What happens as hydrogen atoms move even closer and the overlap increases?
Bond distance
The specific distance between the two nuclei where energy is the lowest and the bond is the most stable one.
Because attractive and repulsive forces are balanced, creating the lowest energy configuration possible.
Why is the bond stable at bond distance?
Repulsions dominate, system energy rises, and the bond becomes destabilized.
What happens if the nuclei moves closer than the bond distance?
The greatest overlap of the p-orbitals (orbitals are directed end to end)
(Also an illustration of the chlorine molecule having a σ bond)
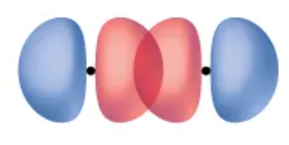
Distance between orbitals and orbital’s orientation
Two things that affects the overlap of the orbitals
s-orbitals, spherical symmetry
_________ does not gets affected at all due to its orientation because of its _________________.
Hydrogen molecule
The molecule where we get to see the overlap of two s-orbitals.
Hydrogen chloride
The molecule where we get to see the overlap of an s-orbital and a p-orbital.
Chlorine molecule
Molecule where we get to see the end-to-end overlap of the two p-orbitals
σ bond
A covalent bond in which the electron density is concentrated in the region along the internuclear axis.
Internuclear axis
A line between the nuclei that pass through the center of the overlap region.
Single bond
Lewis structure’s description of the σ bonds of the valence bond theory
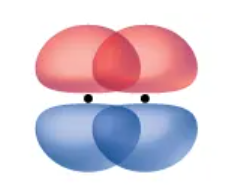
π bond
A type of covalent bond that results from the side-by-side overlap of two p-orbitals.
The regions of orbit overlap lie on opposite sides of the internuclear axis.
Along axis itself, there is a node.
Node
A plane with no probability of finding an electron.
Illustration of a pi bond
1 σ bond and 1 π bond
Double bond of a Lewis structure = ?
1 σ bond and 2 π bonds
Triple bond of the Lewis structure = ?
σ bond
This is the bond that will be the first bond to be formed between any two atoms. And only one bond of this type can exist in any one location
Butadiene
C4H6 (Gets used to make synthetic rubber)
Noble gases
Elements with naturally stable electron configuration; unlike the other atoms, they do no need to form bonds to achieve stability, and octet rule is based on their filled outer shells.
The Concept of The Chemical Bonding
Atoms can attain a stable configuration by exchanging electrons with another atom, resulting in the formation of ions.
Coulombic attraction
A positive-negative charge attraction, a force that causes the ions to associate.
Ionic compounds
The compounds that are formed by the coulombic attraction between the ions.
Covalent bond
A type of bond that is formed when atoms share the electrons instead of exchanging them.
Polar species
The chemical species that are neither completely ionic nor completely covalent.
These chemical species posses a permanent dipole.
Coulomb’s law
The law that describes the force between the two charges equivalent to the product of the charges divided by the square of the distance between them.
F = (Q1*Q2)/(d^2); Q1 = Q2 = 1.602×10^-19 C
Mathematical representation of the Coulomb’s law
E = (Q1*Q2)/d
E = ?
charge, increased distance
The force of attraction increases with the ______ and decreases with the __________________.
Bond dipole moment
A measure of the partial separation of charge between atoms within a single bond due to the differences in electronegativity.
μ = q × d
Mathematical representation of the dipole moment of a bond.
Debye (D)
Unit that is used to measure very small bond dipole moments