Unit 3 - Properties of Substances & Mixtures
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
intermolecular forces
forces holding molecules together
usually affect physical properties (boiling, melting point..)
weaker than intramolecular
Intramolecular forces
forces holding molecule/ionic compound together
covalent/ionic bonds
usually affect reactivity
stronger than intermolecular
Stronger the london dispersion forces the more/less polarizable
more
stronger a molecules intermolecular forces, higher/lower its boiling point
higher
factors that increase LDF’s
high # of e-
high molecular/molar mass
size
more polarizable
higher bp (nonpolar molecules)
low bp = less/more polarizable = less/more e-
less, less
which has higher bp? polar or nonpolar
polar
because dipole dipole attractions in polar are stronger than just LDFs in nonpolar
Hydrogen bonding is a strong type of dipole dipole force found in molecules containing..
O-H, N-H, or F-H
because the molecules bonding with H are extremely electronegative
Why do polar molecules with F, O, N usually dissolve in water?
they can hydrogen bond with water itself
NH3 (g) dissolves in water but PH3 (g) does not… why?
because central atom N is both small and very electronegative causing hydrogen bonds strong attraction to water, but P central atom is larger and less electronegative
what is the strongest force?
ionic forces
so they have the highest mp and bp
as temp increases vapor pressure of a liq ..
increases
temp increases evaporation anyway but when you have a closed lid theres more pressure since container is full of gas
(think of closing the lid on a cooking pot to boil water)
liquids with weak ____ will have higher vapor pressures
weak intermolecular forces
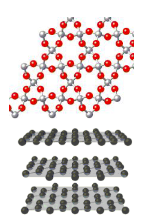
Covalent network solids
extremely strong structure (stronger than ionic compounds) made from REPEATING network of covalent bonds
extremely high mp and bp
strong cov bonds in multiple directions
Ex: diamond, graphite (pure C), silicon dioxide SiO2 (quartz, sand), silicon carbide SiC
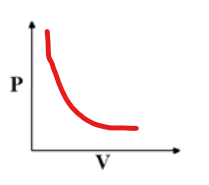
Boyles Law, when to use?
pressure of gas inversely proportional to its volume
P1V1=P2V2
use when temp and moles constant
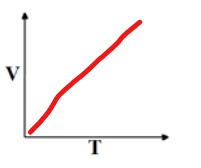
Charles Law, when to use?
The volume of gas is directly proportional to its temp
V1/T1 = V2/T2
temp in kelvin!!
use when pressure and moles constant
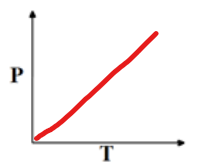
Gay Lussac’s Law, when to use?
The pressure of gas directly proportional to its temp
P1/T1 = P2/T2
t is kelvin!!
use when volume and moles constant
Combined Gas Law
P1V1/T1 = P2V2/T2
use when moles are constant
Ideal Gas Law, when to use?
PV=nRT
use when working with
finding/using moles
mass→ moles conversions
V,P,T all important or given
nothing is changing
Dalton’s Law of Partial Pressures
Partial pressureGas = XGas * PTotal
X is the mole fraction!
(When you have grams in question convert to moles first, then do the formula)
Grahams Law of Effusion, when to use?
Rate of movement of gas particles inversely proportional to their molecular mass
lighter gases move faster, heavier gases slower
use when comparing speed of 2 gases based on molar masses thru effusion/diffusion
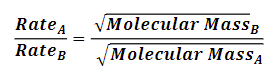
Effusion
the escaping of molecules thru a very tiny hole in a material
Properties of an ideal gas
has molecules that take up no space (no volume)
has molecules with no intermolecular attractions for each other
this shit don’t exist in real world!
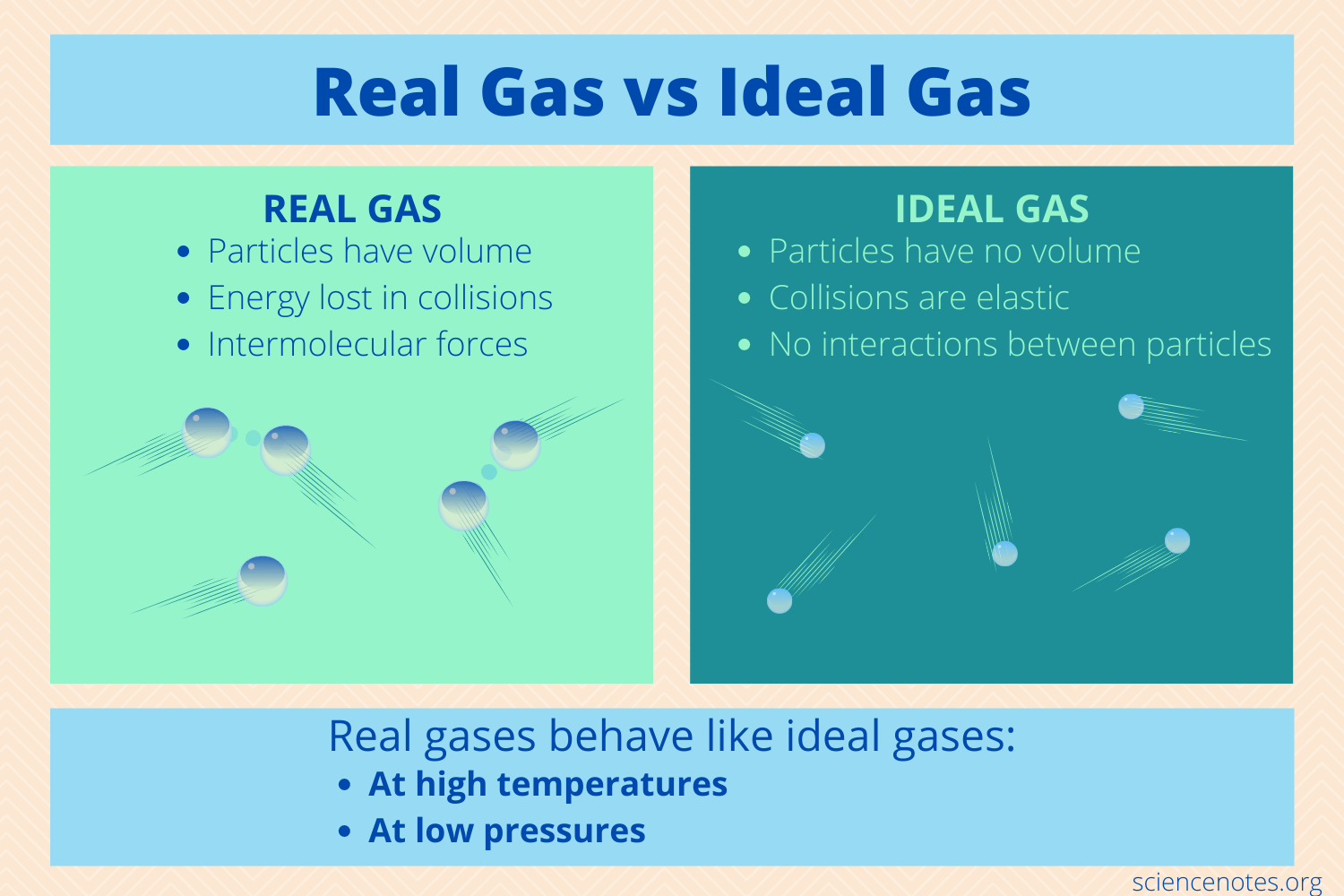
What kind of gases can act like ideal gases?
very small molecules such as He, H2 and Ne that can take up very little space
high temp
low pressure
solvent
medium into which solute is dissolved
water is almost always the solvent
solvent
substance that gets dissolved into a medium
What is a dilute solution?
solution that has lo amnt of solute
opposite of concentrated
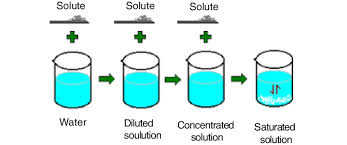
What is a saturated solution?
concentrated solutions that dissolved the max amnt of solute
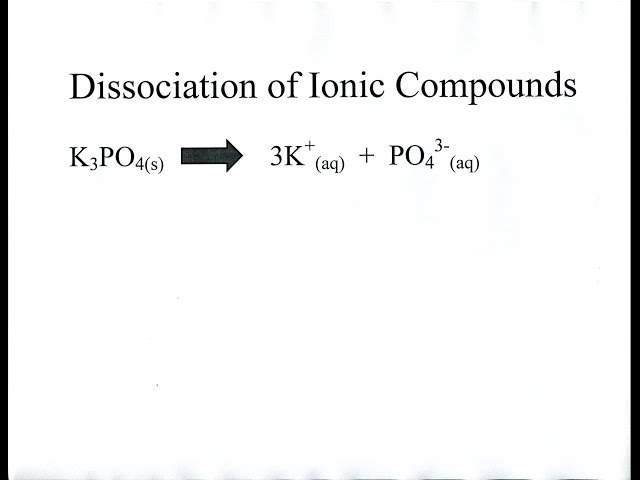
Ionic compounds are strong electrolytes so when dissolved in water they..
dissociate completely into their component ions (can be element ions and/or polyatomic ions)
Like dissolves like
polar molecules like NH3 and C2H5OH dissolve easily in a polar solvent like H2O
nonpolar like CH4 or C6H6 won’t dissolve in H2O!
nonpolar molecules like CH4 and C6H6 dissolve easily in nonpolar solvent like CCl4
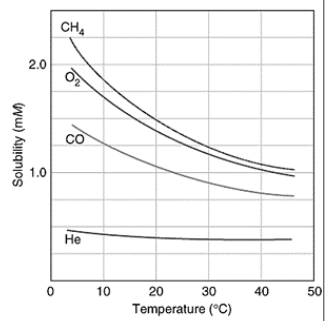
solubility of gas vs temp
as temp increases solubility decreases (inverse)
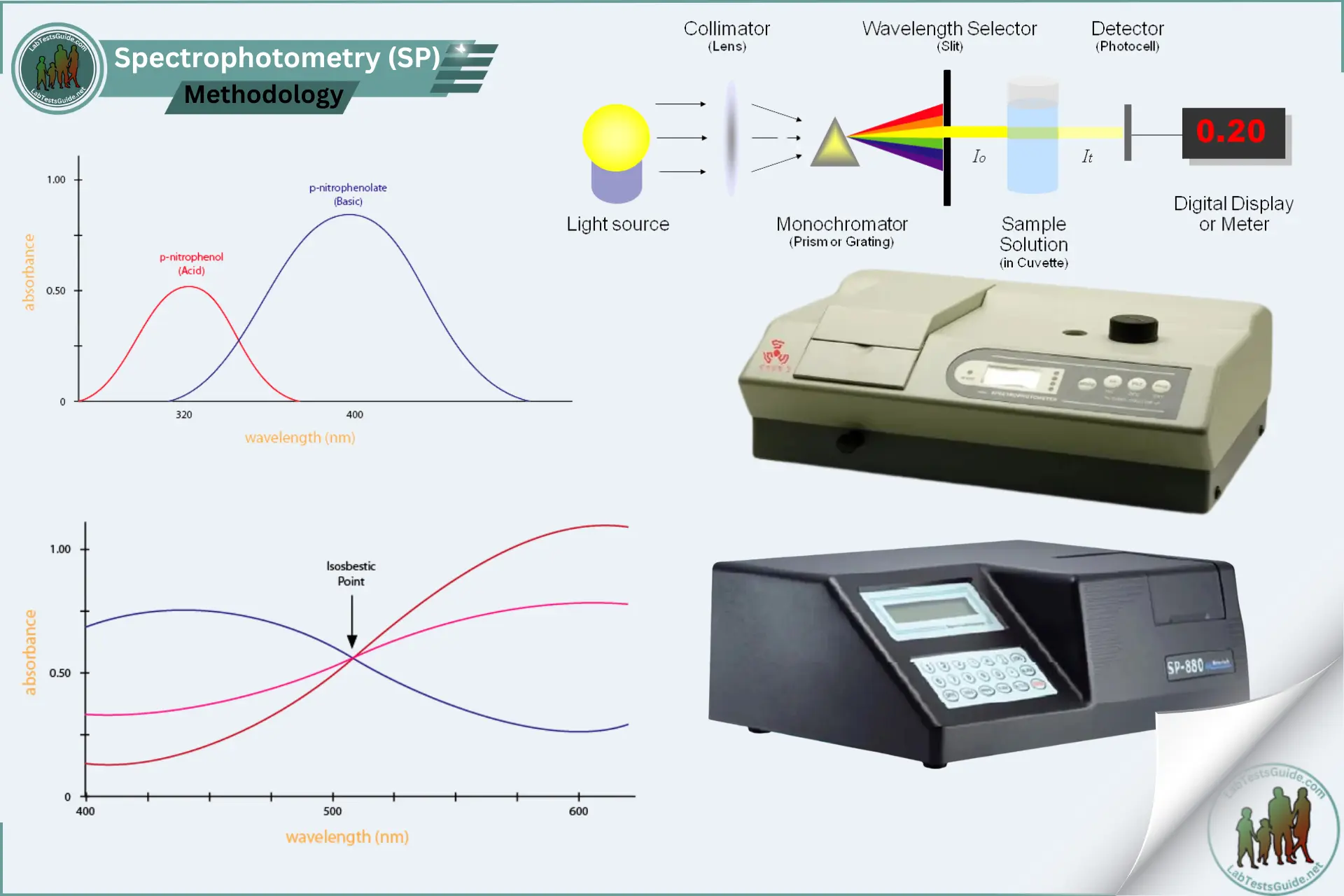
How to determine concentration of a solution using spectrophotometry? (answer in law or formula)
Beer-Lambert Law A = εbc
A= absorbance (between 0-1)
ε= molar absorptivity (constant measuring how well substance absorbs light)
b= path length in cm (width of cuvette, almost always 1cm)
c= concentration (mol/L or molarity)
relationship between absorbance and concentration
directly proportional
since molar obsorptivity and path length are constant
if theres fingerprints on the holder holding a concentration of red dye solution.. how will that affect the absorbance results?
it’s covering some spots so it’s gonna look darker (more concentrated) thus we will think more absorbance (than it actually is..)