PTE 764: quest 1
1/221
Earn XP
Description and Tags
bioenergetics, metabolism, metabolic responses to exercise, pulmonary, cardiovascular
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
222 Terms
what is the study of bioenengetics?
the flow of energy in living things and the take up of that energy stored in chemical bonds and transforming it into mechanical energy (movement)
what is energy?
the capacity to do work and is in a dynamic state that is “measured” with change
what is work?
a measure of amount of force produced and the distance over which that force is exerted
what is power?
the rate (how fast or slow) at which work is done
what is the first law of thermodynamics?
energy can neither be created nor destroyed (however it can be transformed)
what are the two types of energy?
potential energy: the energy an object has due to its position or arrangement within a system, possessing the capability to be converted into kinetic energy
kinetic energy: the energy an object possesses due to its motion; dependent on object’s mass and speed
what are exergonic reactions?
“downhill reactions” that release energy
ΔG is negative = exergonic reaction (catabolism)
what is a side effect of an exergonic reaction?
the generation/release of heat
what are endergonic reactions?
“uphill reactions” that store (potential) energy
ΔG is positive = endergonic reaction (anabolism)
what is the second law of thermodynamics?
in all energy exchanges, if no energy enters or leaves the system, the potential energy of the end state will always be less than that of the initial state
drop in energy = entropy
what is activation energy?
the phenomenon where energy needs to be placed into a state to get energy out of that state
T or F: enzymes containing a dehydrogenase molecule lower the activation energy.
T
organisms need energy to maintain order and life. how does an organism gain more energy?
fueling body through food
reformation of ATP
what does it mean if an organism has high entropy?
the organism ceases to take in energy and dies… RIP
what is one way heat energy may be dissipated?
through sweat
what are the three types of human work?
mechanical work: muscle contractions, mitosis, cilia
chemical work: maintenance and growth of tissues
transport work: moving ions
T or F: the flow of energy defines the limits of exercise intenisty.
T
what are the two roles of enzymes?
accelerate chemical reactions
reduce activation energy
why does the rate of enzymatic activity increase during exercise?
increase in energy demand
increase in intermediate substrates during metabolism
increase in heat generated by body (making faster reactions)
allosteric regulation
why do athletes typically perform warm-ups before events?
it mobilizes enzymes which assists the body in producing the work and power needed
______ can either activate or inhibit an enzyme.
substances
which substance inhibits glycolysis at key enzymatic steps of catabolism?
ATP
T or F: fat burns over a carbohydrate flame.
T
what’s the difference between competitive inhibitors and non-competitive inhibitors?
competitive: mimic substrate by binding to the active site of the enzyme; acting as an on and off switch
non-competitive: bind to an enzyme causing a change in the shape of the molecule and decreases activity
what are the two types of enzymatic reactions?
hydrolysis and condensation
oxidation and reduction reactions (REDOX)
what are the two components of a REDOX reaction?
reducing agent: substance that donates electrons and itself is oxidized
example: lactate
oxidizing agent: a substance that receives electrons
example: pyruvate
how do we measure energy conversion (metabolism)?
heat production
oxygen consumed
carbon dioxide produced
direct calorimetric
measurement of heat production
indirect closed-circuit spirometry
measurement of oxygen consumed
indirect calorimetry: open-circuit
measures oxygen consumption and carbon dioxide production
T or F: ATP is not recycable.
F; ATP must be recycled as the cells have little amounts
what are the four sources of ATP recycle?
readily amounts of ATP in the cytoplasm of the cell
the transfer of phosphate group from phosphocreatine
glycolysis
oxidative pathways
of the four sources of ATP recycling, which one is the ONLY aerobic reaction?
oxidative pathways
where do the three anaerobic sources of ATP occur?
in the cell’s cytoplasm
the aerobic source occurs in the cell’s mitochondria
what is the end product of glycolysis?
pyruvate
once glycolysis is complete, pyruvate can do two processes. what are they?
pyruvate can then either (1) produce more energy through the oxidative pathways or (2) be reduced to form lactate acid in the absence of aerobic metabolism
how do individuals perceive an increase in lactic acid?
a burning sensation
what other two substances (besides pyruvate) can be metabolized through oxidative pathways?
lipids and to some extent proteins
ATP source chart
______ facilitates the entry of glucose into the cell.
insulin
T or F: glucose can enter the cell without insulin.
T
can enter primarily at rest via cortisone
glucose always needs a transporter to enter the cell. what are the two discussed in the notes?
glut-1: not sensitive to insulin (happens at rest; within brain)
glut-4: sensitive to insulin (happens with exercise; within muscle and adipose)
when does the synthesis of glycogen typically occur?
normally stimulated by insulin in response to high serum glucose levels → the elevated glucose levels triggers the cell to synthesize it into glycogen to store it
glycogen synthesis is under the influence of ______ enzyme.
synthetase
when does the breakdown of glycogen typically occur?
when our body is performing activities (need to mobilize our fuel source)
breakdown of glycogen is under the influence of ______ enzyme.
phosphorylase (A)
phosphorylase (B) is the inactive form of phosphorylase (A) so it must undergo conversion B→A for glycogen breakdown to occur. what stimulates this process?
increases in calcium ions and epinephrine which are associated with stress and work
________ is an enzyme from the liver and kidneys that allows glucose-6-phosphate to exit the cell and stimulate more glucose.
phosphatase
occurs when serum glucose is low
glycogen storage disease (GSD)
a group of inherited metabolic disorders that affect the body's ability to store or break down glycogen
you notice in your patient’s chart that he was diagnosed with GSD. what are you expecting from him during treatment?
lack of strength/overall weakness, fatigue, lack of metabolism
McArdle’s Disease
a rare inherited muscle disorder that affects the body's ability to break down glycogen, a stored form of glucose in muscles
how do carbohydrates provide energy for cellular work?
stored energy can form ATP without oxygen
during light-moderate exercise, carbs supply 1/3 of a person’s energy requirements
even usage of large quantities of lipids for energy, there is a requirement for some carb metabolism
metabolism of carbs is rapid
glycolysis is important for activities that take place up to ___ seconds.
90
what are the three general stimulants of glycolysis?
decrease oxygen levels (hypoxia): decrease in Kreb’s cycle and oxidative phosphorylation
increase in ADP and Pi levels: energy starved
increase calcium inside the cells: contracting skeletal muscle
what is the bottleneck (limiting) enzyme for glycolysis?
phosphofructokinase (PFK)
increase of activity of PFK during maximal exercise to control glycolysis
what are the four factors that inhibit PFK?
ATP
citrate
low pH
glucagon
what activates PFK?
ADP and AMP (high energy demands)
the dephosphorylization of _________________ produces ATP (gives us energy).
phosphoenolpyruvate (PEP)
pyruvate kinase is the enzyme responsible for converting PEP into pyruvate. what can inhibit this conversion?
alanine (an amino acid that signals cell of too much pyruvate)
following glycolysis, what happens to the extra NADH?
either (1) continues through fast/anaerobic process to assist pyruvate in creating lactate or (2) continues through slow/aerobic process to help with oxidation via ETC and Kreb’s cycle to produce more ATP
NADH requires a transporter to enter into a cell’s mitochondria. what are the two transporters/shuttles?
malate-aspartate shuttle: for heart and liver cells
glycerol-phosphate shuttle: for skeletal muscle and brain cells
a feed-forward system gets a process moving. what are two examples of feed-forward regulations for glycolysis?
glycogenolysis: stimulated via epinephrine and muscle contractions
glucose uptake: stimulated via insulin and muscle contractions
a feedback system inhibits or slows a process. what are some examples of feedback regulations for glycolysis?
phosphofructokinase (PFK)
cellular energy charges
lactate de-hydrogenase
pyruvate de-hydrogenase
cytoplasmic redox potential
LDH competes for pyruvate when glycolysis is slow. describe the two types of LDH we discussed?
M (muscle and anaerobic) type has a high affinity for pyruvate
H (heart and aerobic) type has a low affinity for pyruvate
but has a high affinity for lactate and can actually increase pyruvate
what are the two forms of lipase?
lipoprotein lipase: promotes storage of lipids; stimulated by insulin and glucose
hormone sensitive lipase (HSL): promotes the release of lipids from adipose tissue; stimulated by epinephrine, norepinephrine, and growth hormone
what are the seven steps of lipid metabolism?
mobilization
circulation
uptake
activation
translocation
beta-oxidation
mitochondrial oxidation
the key point of mobilization of a lipid (free fatty acid) is to get it into the blood’s circulation. how does that happen?
free fatty acid (FFA) must be bound to albumin to transport through the blood
translocation of a FFA involves stripping of “original” CoA, transporting activated FA into the mitochondrial matrix, and then rebinding a “new” CoA. what co-enzyme is needed for this process?
carnitine
T or F: carnitine can be recycled.
T
carnitine deficiency may result in or be present in….
myopathy
hypoglycemia
cardiomyopathy
T or F: glucose produces more ATP than lipids.
F; lipids produce more ATP but glucose is more efficient and faster
what is the feed-forward regulation of lipid metabolism?
circulating FFA level is controlled by adipose lipolysis rate under hormone sensitive lipase (HSL) regulation
HSL is usually epinephrine
what is the feedback regulation of lipid metabolism?
acetyl CoA inhibits beta-oxidation; signaling to the body that Kreb’s cycle is doing well
if the Tricarboxylic Acid Cycle (TCA)/Kreb’s Cycle is not the main energy producing reaction within the body, why is it important for it to occur?
it gives off reducing equivalences (FAD and NADH2) which go to other reactions/cycles to create ATP there
what three sources produce acetyl CoA for the kreb’s cycle?
from pyruvate (CH2O)
from fatty acyl CoA (lipids)
from amino acids (proteins)
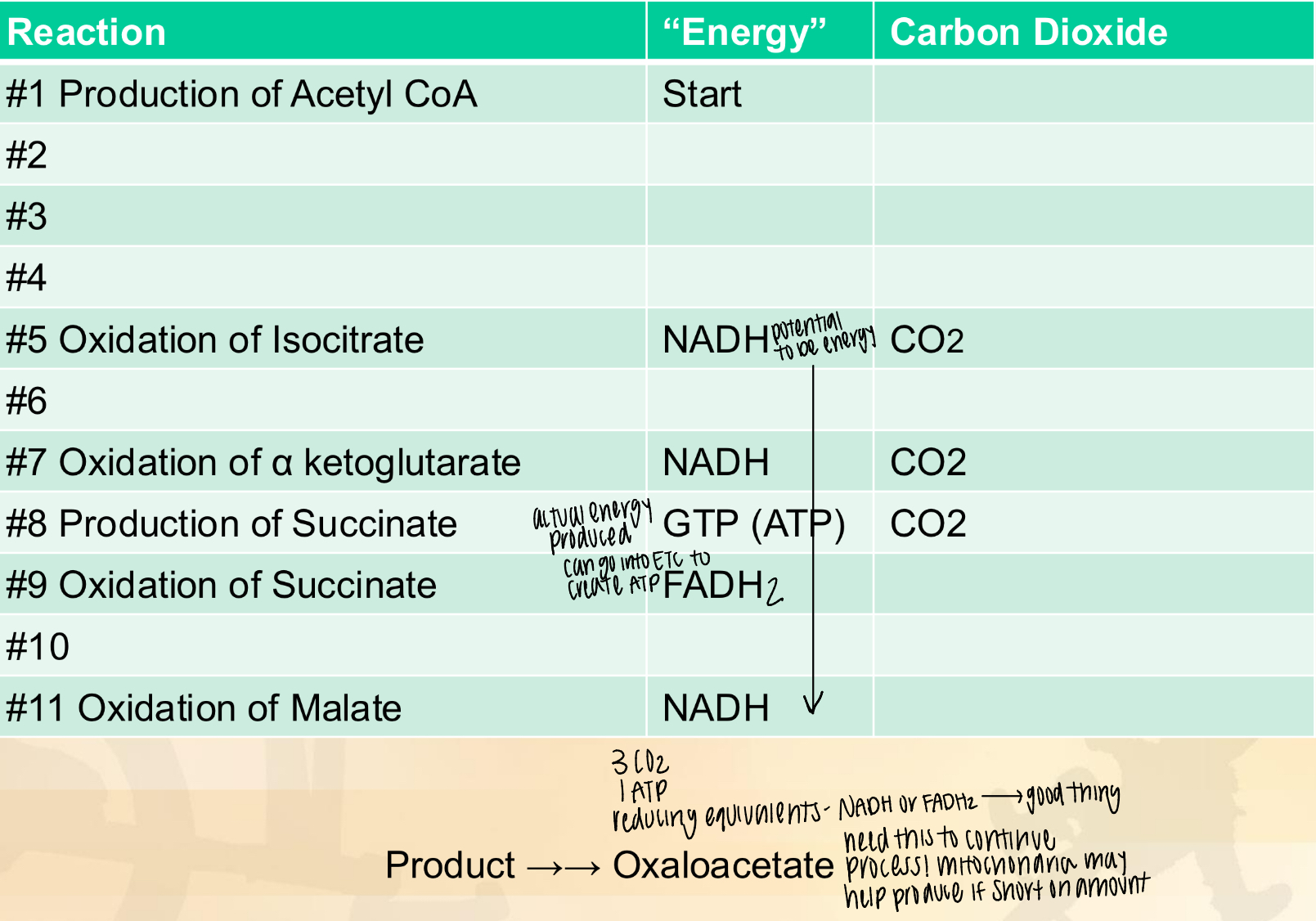
TCA: oxidation of isocitrate
substrate: isocitrate
enzyme: isocitrate dehydrogenase
product: oxalosuccinate
oxidizing agent: NAD+
NAD+ is reduced to NADH + H
production of CO2
TCA: oxidation of alpha-ketoglutarate
substrate: alpha-ketoglutarate
enzyme: alpha-ketoglutarate dehydrogenase
product: succinyl CoA
oxidizing agent: NAD+
NAD+ is reduced to NADH + H
production of CO2
TCA: production of succinate
substrate: succinyl CoA
enzyme: succinyl kinase
product: succinate
synthesis of ATP
production of CO2
TCA: oxidation of succinate
substrate: succinate
enzyme: succinte dehydrogenase (SDH)
product: fumarate
oxidizing agent: FAD+
FAD+ is reduced to FADH2
TCA: oxidation of malate
substrate: malate
enzyme: malate dehydrogenase
product: oxaloacetate
oxidizing agent: NAD
NAD+ is reduced to NADH + H
tally of Kreb’s Cycle/TCA chart
what are the feed-forward regulations of TCA/ Kreb’s cycle?
production of acetyl CoA
production of oxaloacetate
what are the feedback regulations of TCA/ Kreb’s cycle?
isocitrate dehydrogenase (bottleneck/limiting enzyme)
cellular energy charge
mitochondrial redox potential
what is the ATP count associated with the reducing equivalences at the end of the electron transport chain (ETC)?
NADH = 3 ATP
FADH = 2 ATP
when studying metabolism during exercise, what is considered the steady state time frame?
1-4 mins
what are the three events occurring at the steady state?
energy requirements are met by aerobic metabolism
a state of inadequate oxygen consumption exist at the tail end of the lag period; have an oxygen debt
the fuel used from rest to light exercise for aerobic metabolism is glycogen (glucose)
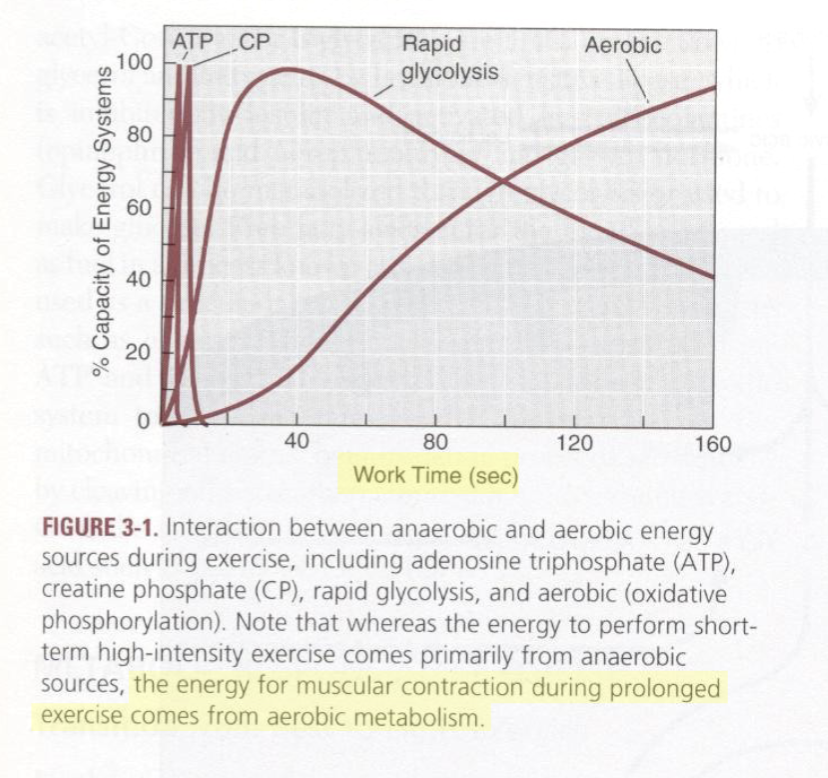
what time frame dictates a short term high intensity exercise?
5-60 secs
activities under 5 secs: energy from ATP-CP system
activities around 30 secs: energy from combo of ATP-CP and anaerobic/rapid glycolysis
short term high intensity exercises mainly utilize the substrate _______.
carbohydrates
although the exercise duration is “short”, why does blood lactate continuously rise throughout exercising?
lactic acid is the by-product of anaerobic activity (glycolysis) so as the activity continues so will the production of lactic acid
what occurs at the anaerobic/lactate threshold?
the body has saturated it’s anaerobic systems (pyruvate and O2) so it will switch to aerobic systems
what does the anaerobic/lactate threshold tell us?
how aerobic a person is
why does anaerobic threshold vary between individuals?
trained vs. untrained, age, lifestyle habits, etc.
T or F: anaerobic glycolysis exists when the hydrogen (NADH) produced is oxidized at about the same rate as it becomes available meaning production = utilization.
F; its aerobic glycolysis
what is the final product of aerobic glycolysis?
pyruvate
steady state sub VO2max can be maintained during 10-60 mins of sub-max continuous exercises. what are the two exceptions to this statement?
hot, humid environments
continuous exercise at a relatively high workload
while exercising in the above environments (hot, humid, and high intensity), the body experiences a slow rise in VO2 due to an increase in serum catecholamines. what do these do?
stimulate lipolysis (HSL) → increases FFA
stimulate the breakdown of glycogen (phosphorylase) → increases serum glucose
may release glucagon in the presence of depleted serum glucose levels
prolonged sub-max exercises mainly utilize the substrates ___ and ____.
lipids and carbohydrates
during low to moderate intensity exercise there is a shift form carbs to fatty acids as the prime substrate for metabolism. how does this occur?
fatty acids stimulate Krebs cycle → increases citrate → feeds back to lower PFK activity → lowers the oxidation rate of glucose
what three measurements are useful parameters to predict the success of an athlete?
VO2max
lactate/anaerobic threshold
changes in ventilation (respiratory rate and depth)
the terminal acceptor for hydrogen in the electron transport chain is ____.
oxygen