maxwell-boltzmann distributions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
what must occur for a reaction to take place?
a collision in the correct orientation w/ a minimum amount of EK (the EA)
draw the general shape of a Maxwell-Boltzmann distribution:
what is the area under a Maxwell-Boltzmann distribution = to?
total number of molecules
why does the curve of a Maxwell-Boltzmann distribution start at 0?
no molecules have 0 energy
what does the peak of a Maxwell-Boltzmann distribution curve represent?
most likely energy of any single molecule
where is the mean energy of all the molecules on a Maxwell-Boltzmann distribution curve?
a point slightly right to the peak/most likely energy
where is the EA on a Maxwell-Boltzmann distribution curve?
(towards bottom of curve on RHS)
how will the shape of the Maxwell-Boltzmann distribution change if the temperature is increased? why?
curve shifts to right and peak lowers (area stays the same as total no. of molecules is =)
because a greater proportion of molecules have at least the EA and are able to react
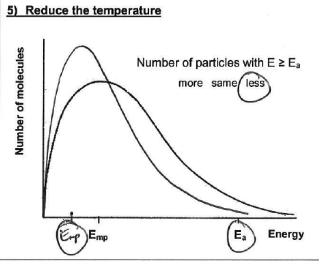
how will the shape of the Maxwell-Boltzmann distribution change if the temperature is decreased? why?
curve shifts left and peak gets higher (area stays the same as total no. of molecules is =)
because a fewer proportion of molecules have at least the EA and are able to react
how will the shape of the Maxwell-Boltzmann distribution change if the concentration is increased? why?
peak gets higher - area increases
more molecules available to collide in a given volume and more molecules w/ energies > EA
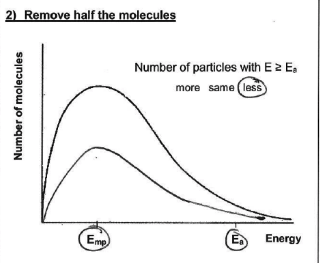
how will the shape of the Maxwell-Boltzmann distribution change if the concentration is decreased? why?
peak gets lower - area decreases
fewer molecules available to collide in a given volume and fewer molecules w/ energies > EA
how will the Maxwell-Boltzmann distribution change w/ the addition of a catalyst? why?
no change in shape
but EA lowered so moves slightly left of curve
as more molecules have energies at or higher than EA