BioChem Exam 3 Content
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
What is the structure of a fatty acid?
How many number of carbons are present in fatty acids (even/odd)?
What does the position of the double decide?
Hydrocarbon (just C’s and H’s) with a carboxylic acid at the end
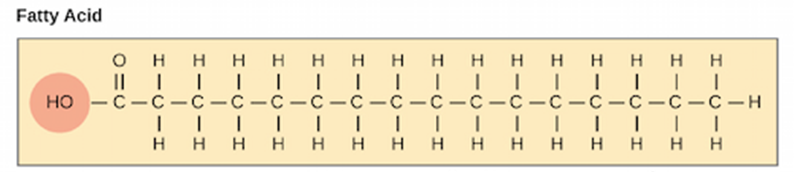
Even number of carbons, usually 16 to 18 carbon atom chains
(Note: The methyl carbon atom on the far right end of the structure is called the omega carbon)
The number of what type of Omega fatty acid it is (Omega-3, Omega-6, Omega-9)
What is the structure of a fat molecule?
What is this structure linked together by?
A glycerol backbone with three fatty acid tails
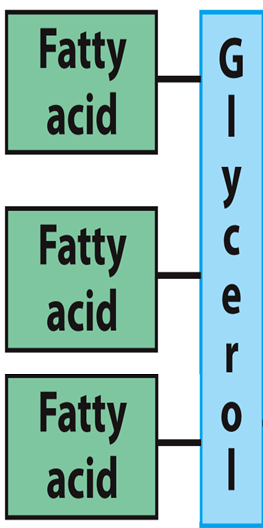
Ester linkages
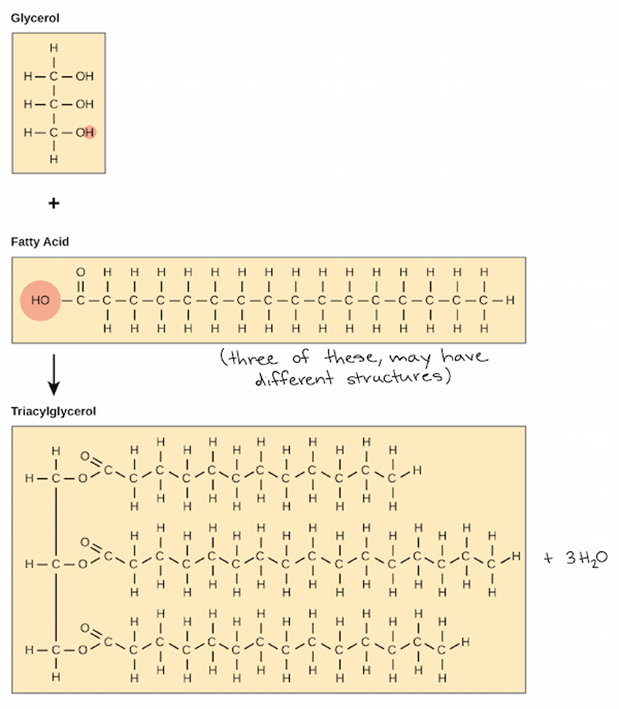
(Note: Ester linkages change the carboxylic acid group into ester group with a by-product of 3 water molecules)
What is the structure that increase the risk of heart disease and stroke, including obesity and metabolic syndrome?
Triglycerides
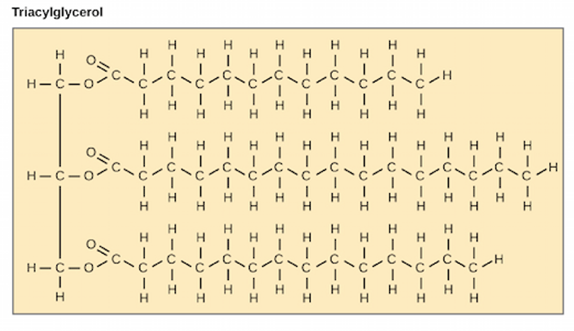
(Note: TGs are super hydrophobic)
What are the four total components of a phospholipid?
What are the two common platforms for phospholipids?
Fatty acids (1 or more), a platform, a phosphate, and an alcohol
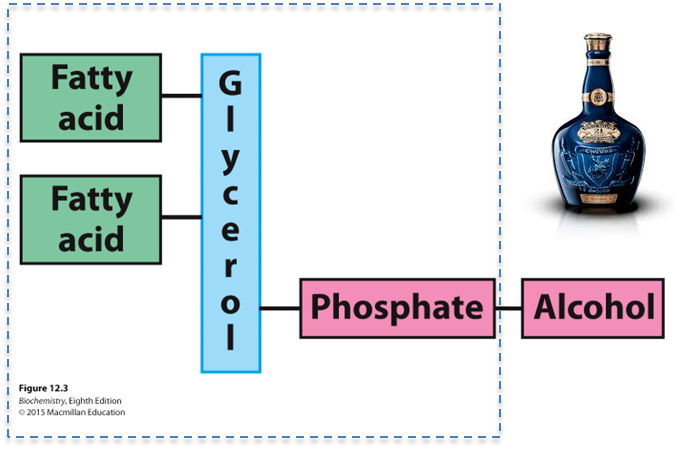
Glycerol and sphingosine
What is the structure of a phosphatidate?
What is phosphatidate (PA) a precursor for?
A glycerol backbone, fatty acids on carbons 1 and 2, and a phosphate on carbon 3
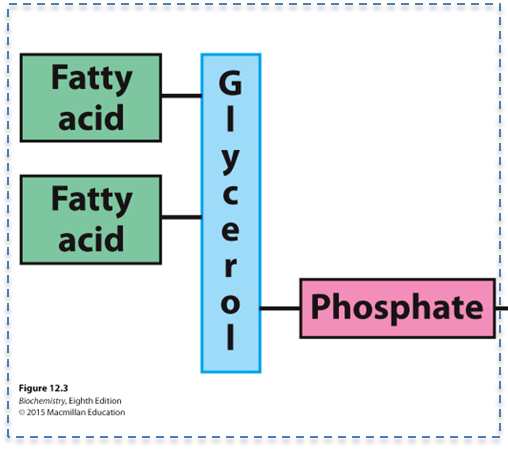
The biosynthesis of many other lipids
What are the two most important features of the biological membrane?
Non-covalent assemblies
Asymmetric
How is the membrane asymmetric?
The outer surface (hydrophilic heads) are different from the inner surface (hydrophobic tail)
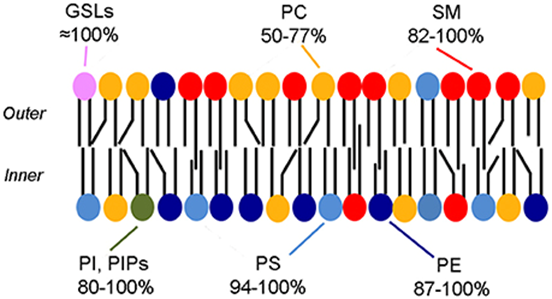
(Note: The different shapes of the black lines, which are the hydrophobic tails)
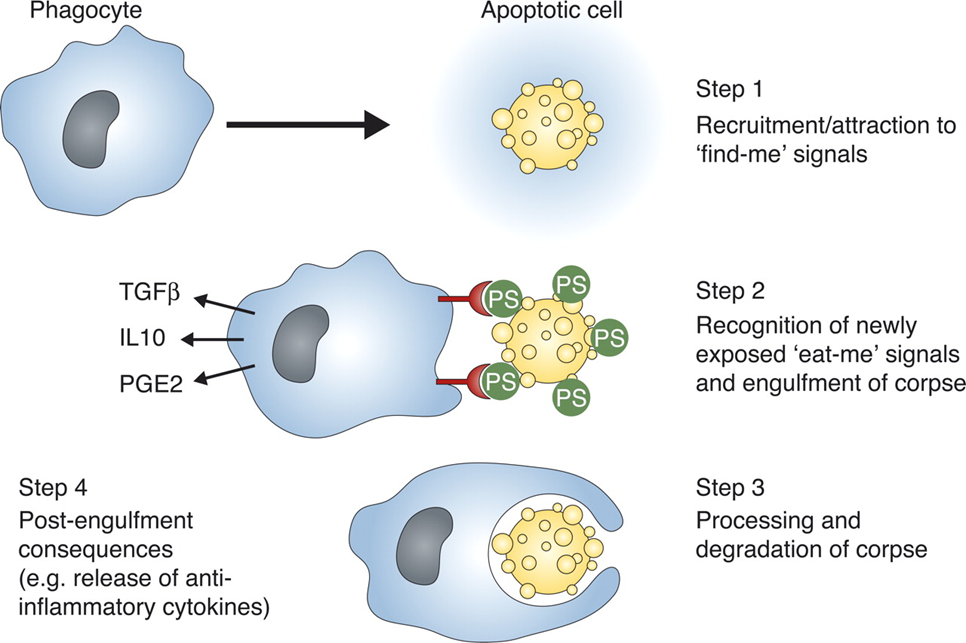
What is apoptosis?
Programmed cell death (peaceful cell death)
Where is phosphatidylserine (PS) kept in?
Where can PS be exposed?
What is implied by the “eat me” signal?
The inner membrane (the side facing the cytoplasm) in healthy cells
At the cell-surface of dying, mutated, cancerous, or microbe-infected cells
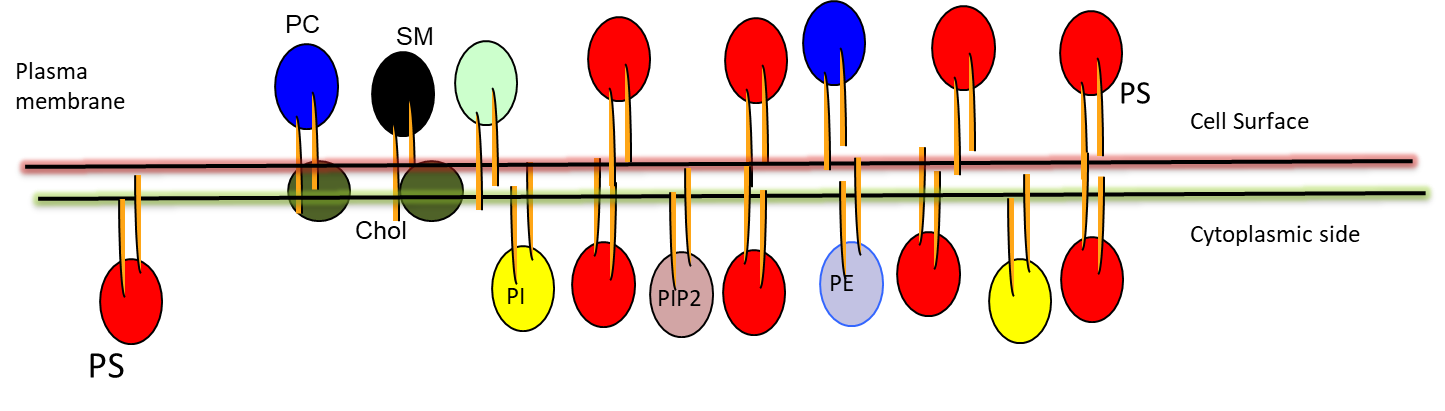
PS exposure serves as an “eat me” signal so macrophages can hunt it out (only exposed in bad cells)
What is the structure of a sphingolipid?
Where are they located and what are they responsible for?
Sphingosine, an amino alcohol that contains a long, unsaturated hydrocarbon chain
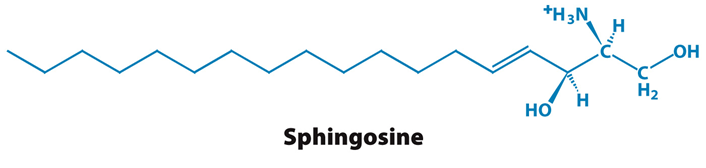
(Note: Instead of a glycerol backbone)
In nervous tissue and responsible for cell signalling
What are glycolipids?
What type of bond are they connected by?
Lipids with carbohydrates (sugar containing lipids) attached
Covalent glycosidic bond
What group in the cholesterol structure interacts with phospholipid head group?
The hydroxyl group
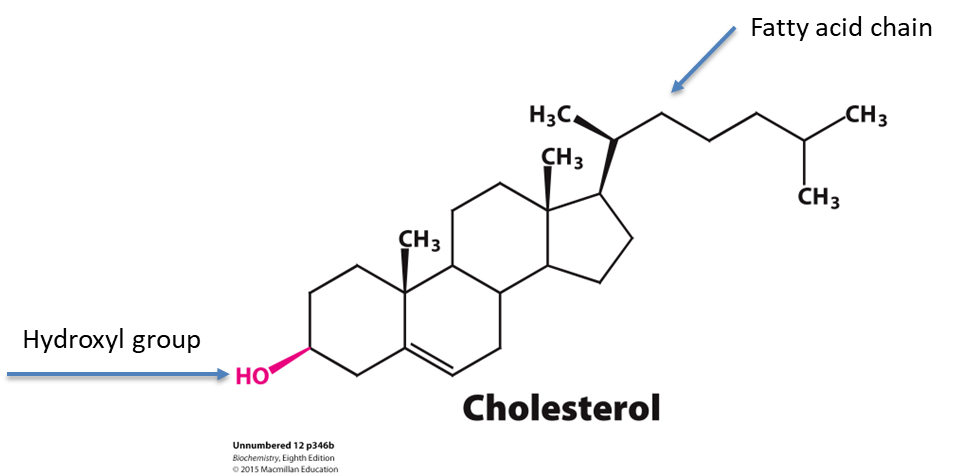
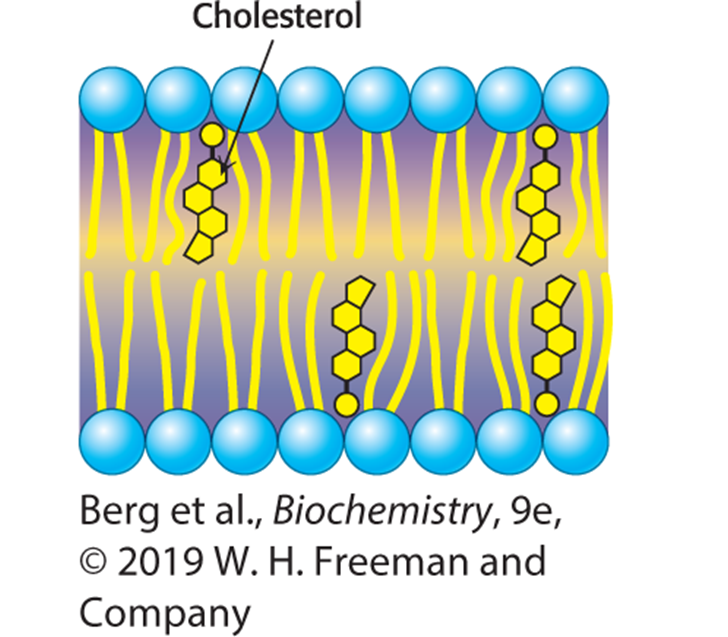
What type of regulation does cholesterol provide?
Bidirectional regulator of membrane fluidity
(Can insert cholesterol when membrane is too molten OR too rigid)
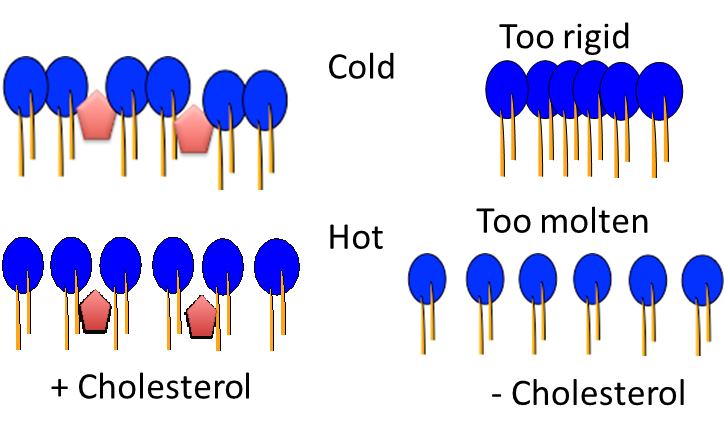
What does cholesterol form complexes with (3 things)?
What are lipid rafts? What can they function in?
Why do many pathogens use lipid rafts?
Sphingolipids, glycolipids, and some GPI-anchored proteins
A “door” to acknowledge the signal and send the signal to go to further pathways, not letting it in. Functions in signal transduction
As a portal to enter cells because they contain structures that the substrate recognizes (“lock and key”)
Where is cardiolipin most often found?
What is the enzyme required for the proper synthesis and maintenance of cardiolipin?
What is barth syndrome? Symptoms?
In the membranes of bacteria, archea, and the inner membranes of mitochondria
Tafazzin
Mutations that reduce the catalytic activity of tafazzin. Symptoms include dilation of the heart chambers, exercise intolerance, and impaired growth
How are membranes made permeable to the lipid bilayer (3 things)?
What are ion channels?
Why do pumps use ATP for active transport? Give two examples of ATP-driven transporters.
Channels, carriers, and pumps
Pore-forming membrane proteins
To transport substrates against the concentration gradient (low —> high). P type ATPases and ABC (ATP-binding cassette) transporters
What kind of molecules can pass membranes down the concentration gradient through simple diffusion (high —> low)?
What kind of molecules require the presence of a channel to move across a membrane down their concentration gradient through facilitated diffusion/passive transport?
Lipophilic (lipid-loving, hydrophobic)
Charged or highly polar molecules (ex: Na+)
What determines the direction in which steroid hormones can move through a membrane?
List two other general examples that can pass through a membrane.
Concentration gradients
CO2, O2, ions
What does the N domain serve as in a P type ATPase pump mechanism?
What does the P domain serve as in a P type ATPase pump mechanism?
What does the A domain serve as in a P type ATPase pump mechanism?
Built-in protein kinase that functions to phosphorylate the P domain (add phosphate)
Receives the action to either phosphorylate or dephosphorylate
Built-in protein phosphatase that functions to dephosphorylate the phosphorylated P domain (take away phosphate)
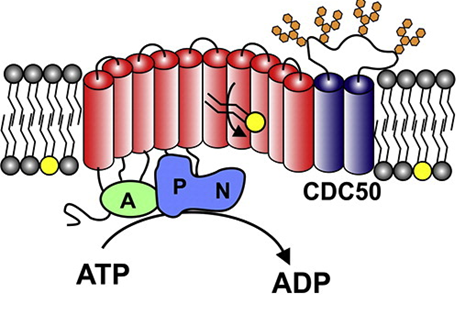
What type of steroid is used to treat congestive heart failure?
What does this steroid do?
Where can this steroid be found?
Cardiotonic steroids
Inhibit sodium potassium ATPase by blocking the sodium potassium pump’s ability to dephosphorylate, increasing the calcium in the body for better contracting of the heart
Foxglove flower (Digitalis purpurea)
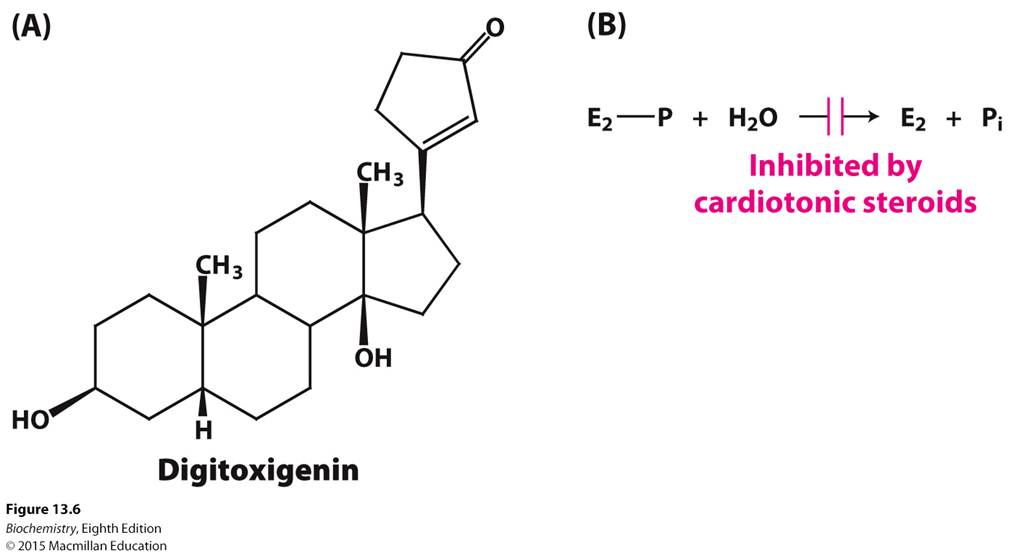
(Note: Digitoxigenin is an example of the cardiotonic steroid that inhibits dephosphorylation)
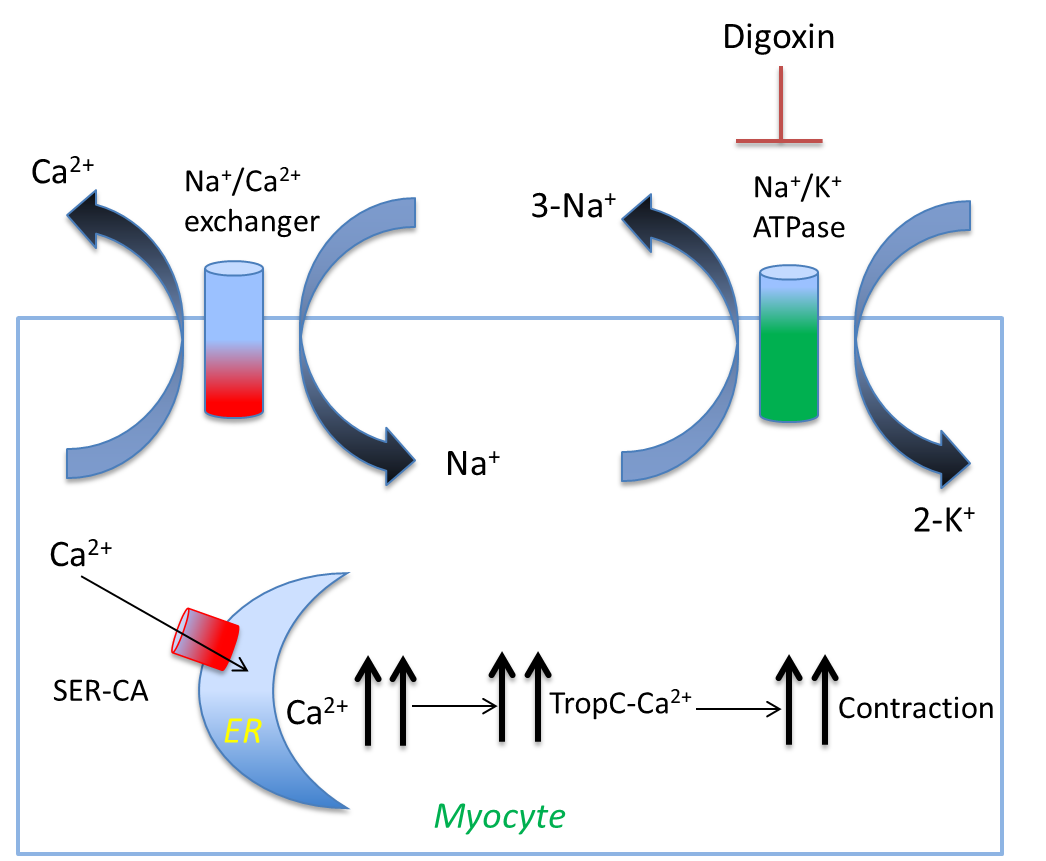
List the four steps that results when the sodium/potassium pump gets blocked or stops working.
Sodium/Potassium pump stops working/blocked, resulting in excess Na+ inside of the cell
Sodium/Calcium pump stops working, resulting in excess calcium inside of the cell
SER-CA moves the excess calcium into the ER
Muscle contraction
List the two names of the multidrug resistance microbes.
(Note: Look at Physical flashcard!!)
Methicillin-resistant Staphylococcus aureus (MRSA)
Multidrug-resistant Mycobacterium tuberculosis (MDR-TB)
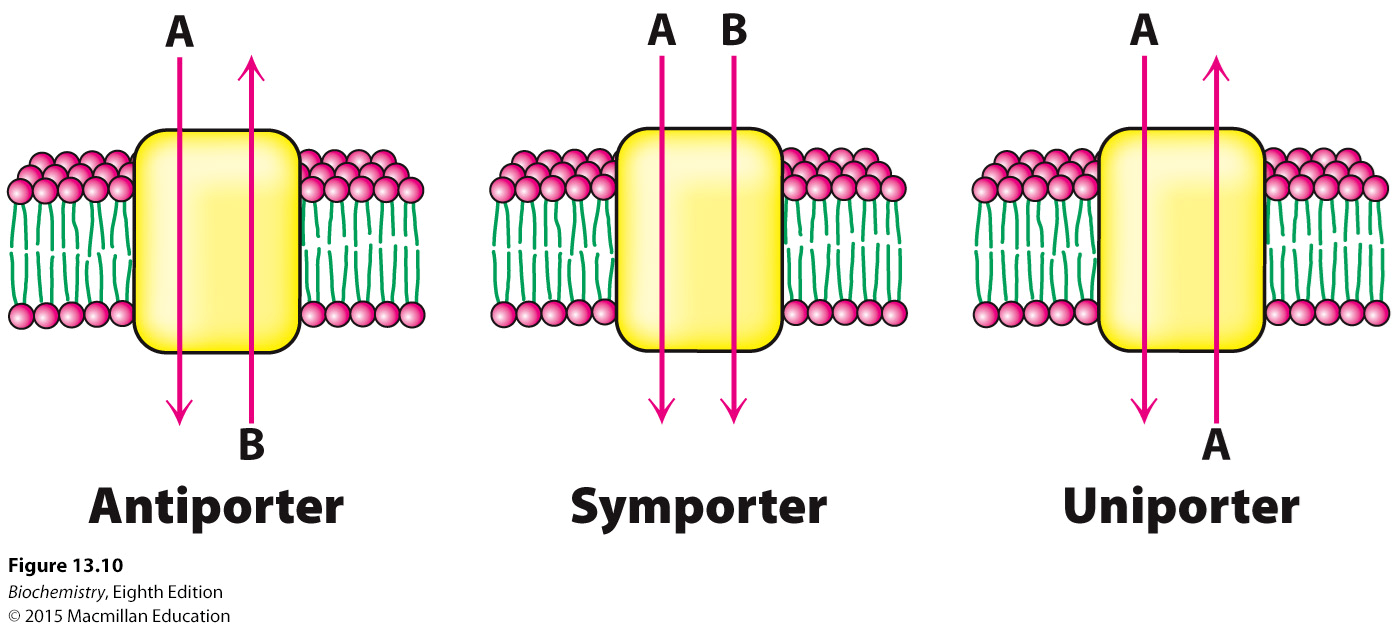
What are antiporters? Give an example.
What are symporters? Give an example.
What are uniporters? Give an example.
Two different molecules, one goes in, the other goes out (opposite direction). Sodium/Calcium pump
Two different molecules, both go in or both go out (same direction). Sodium/Glucose transporter
One molecule that can go in or out. Glucose transport (GLUT)
If all of the pumps’ mechanisms are the exact same, how can they transport different ions?
What is potassium’s specific substrate?
What is calcium’s specific substrate?
What is sodium’s specific substrate?
Specificity: How channels are specific to its substrate
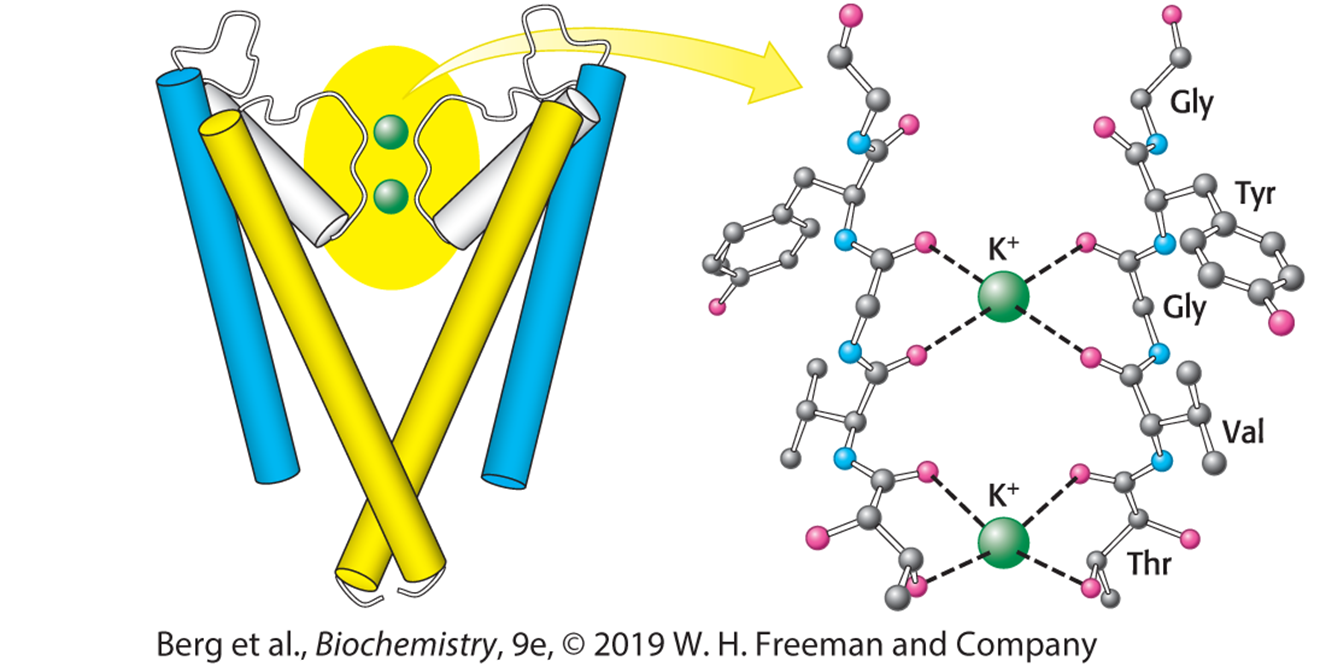
(Note: Potassium’s specific substrate is the TVGYG sequence of the electivity filter)
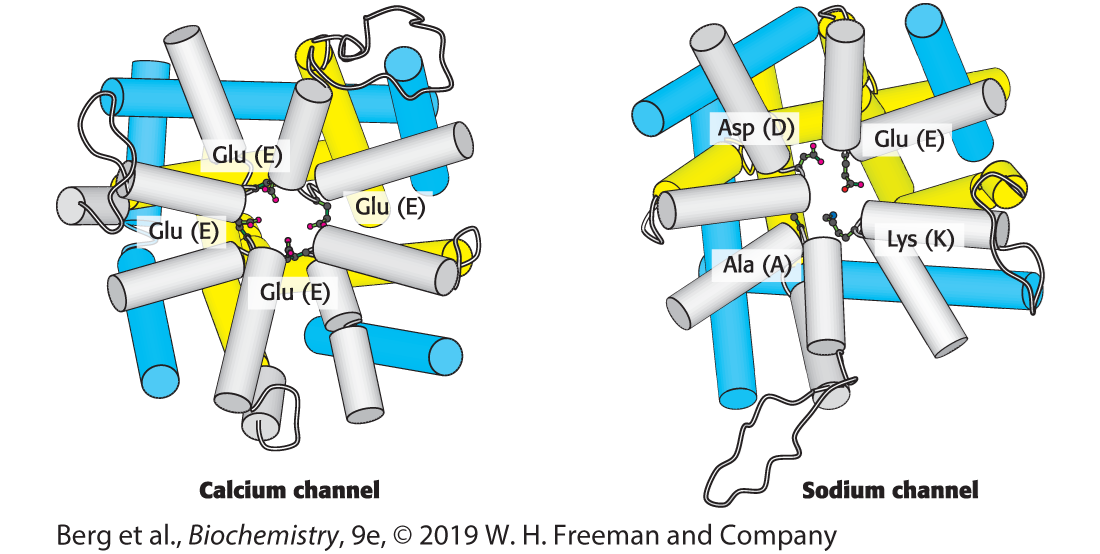
(Note: Calcium’s specific substrate is Glutamic acid)
(Note: Sodium’s specific substrate is DEKA)
What is the acetylcholine receptor classified as?
Sodium-potassium channel. When the ligand acetylcholine binds to the receptor, the channel opens and allows sodium and potassium to enter the cell
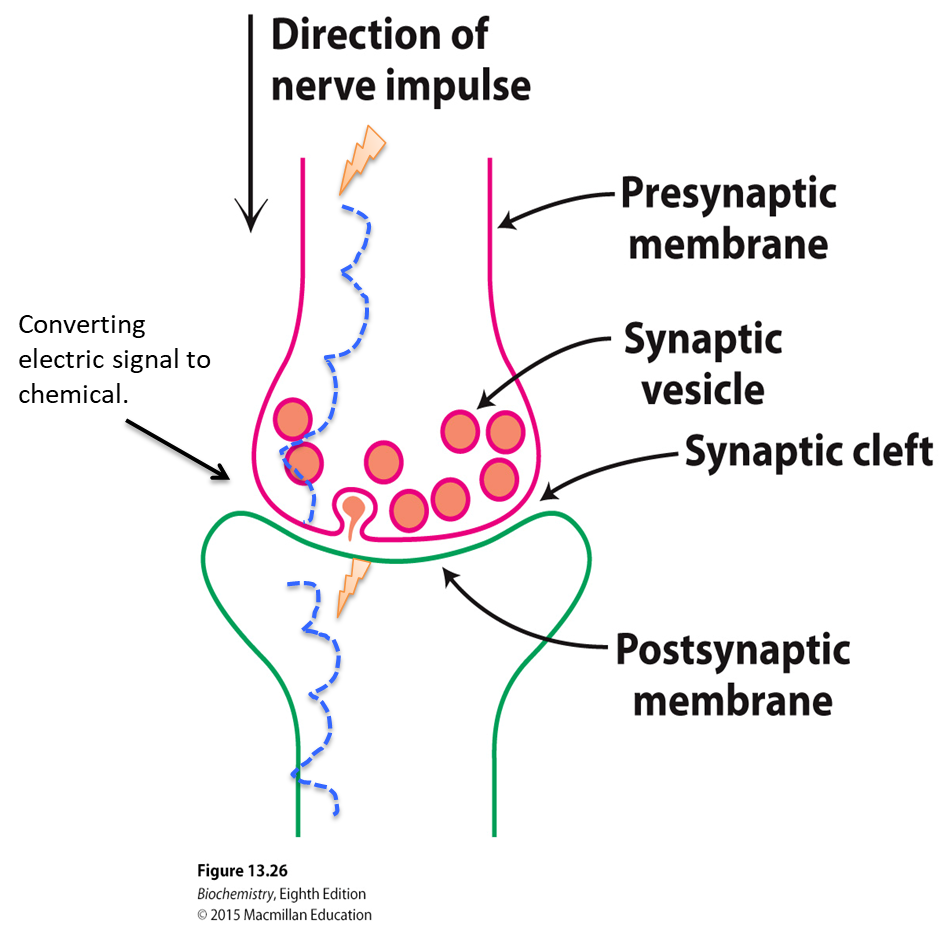
(Note: Binding of acetylcholine causes conformational changes that rotate the membrane-spanning helices so that the pore opens to the passage of sodium and potassium ions)
What is Long QT syndrome (LQTS)?
What can this lead to?
What is the most common mutation identified?
A genetic disorder in which the recovery of the action potential from its peak to the resting equlibirium is delayed
Brief losses of consciousness (syncope), disruption of normal caridac rhythm (arrhythmia), and sudden death
Inactive potassium channels or prevention of the proper trafficking of these channels to the plasma membrane
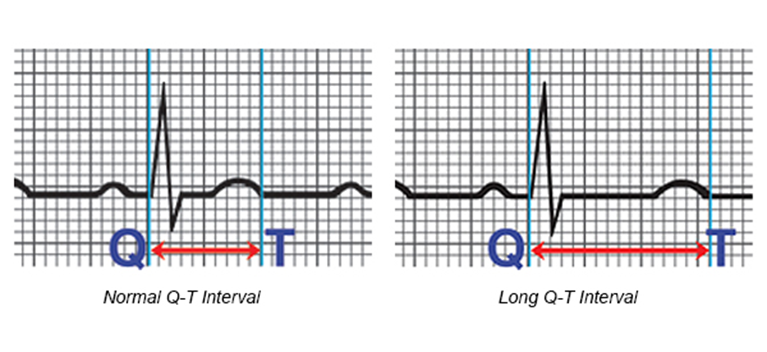
(Note: Graph represents the time it takes for the heart muscle to contract and then recover)
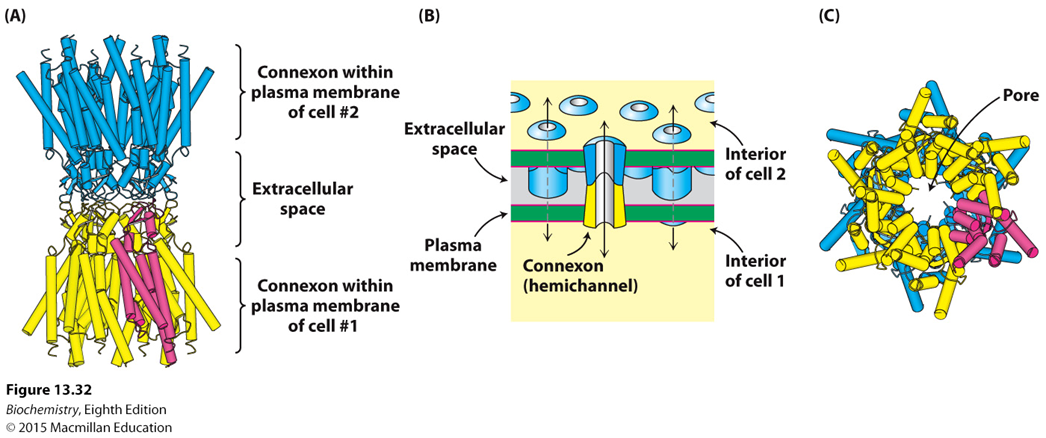
What are gap junctions and what do they generally allow?
What forms gap junctions? How many do these take to make a cell-to-cell junction?
What type of communication do gap junctions allow?
Cell-to-cell channels that allow passage of material between adjacent cells
Connexons from adjacent cells. 12
Cytoplasm-to-cytoplasm communication
What are the three types of receptors for signal transduction?
G-protein coupled receptors (GPCR)
Enzyme-coupled receptors
Ion-channel receptors
How many transmembrane receptors are always associated with G proteins?
About how many approved drugs target GPCRs? What % of approved drugs does this account for?
List three examples of the types of GPCR approved drugs.
7 (7TM)
~700. 35%
Oxytocin, Xanax, Oxycodone
List three common secondary messengers for signal transduction.
cAMP, Calcium, and IP3 (Inosital 1,4,5-triphosphate)
What does Cyclic AMP (cAMP) activate?
Protein Kinase A (PKA)
(Hence why PKA is also known as cAMP-depdendent protein kinase)
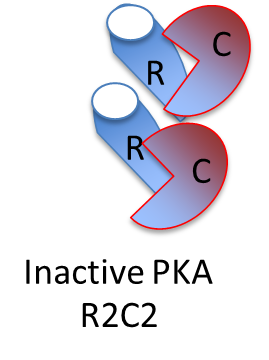
What type activity of a G protein terminates a signal?
What is this an example of?
GTPase activity
Bad enzyming (breaking down the same thing which activates them)
What is one of the main things used to bind to a receptor and terminate a signal?
Beta-Arrestin (or B-arrestin) binds to the phosphorylated receptor and terminates the signal
What does PLC do in the Phosphoinositide Cascade?
Cleaves PIP 2 into two secondary messengers: IP3 and DAG
What is the name of the protein that is a common calcium (Ca 2+) sensor?
What is the trigger for this to bind to Ca 2+?
Calmodulin
When calcium concentration in the cytoplasm increases (gets too high)
What kind of structure is the insulin receptor?
What are the two subunits of the insulin receptor bound together by?
A dimer (even in the absence of insulin)
Disulfide bond
What three things does IRS-1 bind together at once?
What is the PTB domain?
What is the PH domain?
What is the SH2 domain?
(Note: Look at Physical Flashcard of Insulin pathway!!)
Insulin receptor, PIP2, and PI3K
Where the head of the IRS-1 binds to the beta-unit phosphate (phospho-tyrosine)
Where the smaller head of the IRS-1 binds to PIP2
Where the head of the PI3K binds to the series of phosphates on the tail of IRS-1
What is PTEN?
A type of lipid phosphatase that breaks down PIP3 to PIP2
What are the two most important physiological stimuli to increase glucose transport into skeletal muscle fibers?
Exercise and Insulin
What structure does EGFR (EGF Receptor) exist as?
When does dimerization occur?
What type of suppressor is it when some of the phosphatases terminate an EGF signal?
Monomer
When each monomer has a bound EGF
Tumor suppressors
Mutated or overexpressed normal version of … are found in many tumors. List two examples of what these might be.
What has been generated to inhibit the signalling pathway?
Receptors. Tyrosine Kinase Receptors, 7TM Receptors
Monoclonal antibodies
True or False? Glycolysis is a metabolic pathway that requires Oxygen.
Where does glycolysis occur?
False. Glycolysis is a metabolic pathway that does not require Oxygen
In the cytoplasm
What three things can glucose be converted to in the liver?
ATP, fat, glycogen (liver glycogen is broken down into glucose and sent through the blood stream as fuel for other organs like the brain)
What is the only fuel that RBCs are able to use at all?
Glucose
What are the three irreversible enzymes used in glycolysis?
Hexokinase
Phosphofructokinase (PFK)
Pyruvate kinase (PK)
What is it called when one structure has a greater phosphate-transfer potential than ATP and thus can be used to power the synthesis of ATP from ADP and Pi, and is catalyzed by phosphoglycerate kinase?
Substrate-level phosphorylation
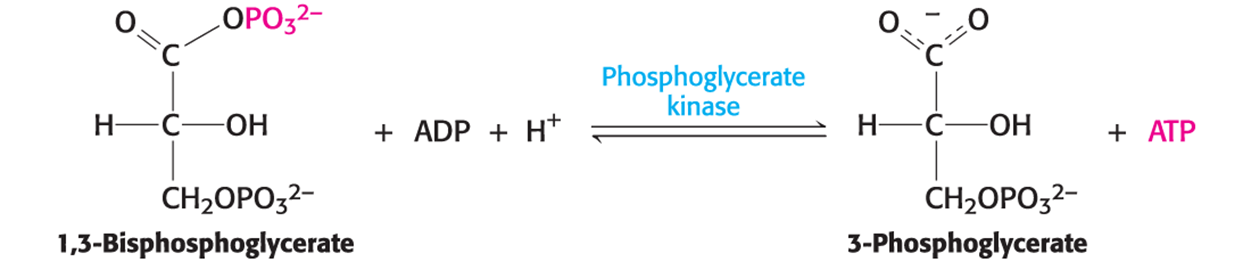
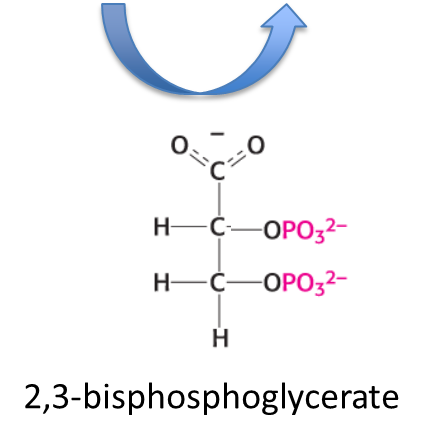
What does 2,3-BPG do?
Binds to hemoglobin and decreases its affinity of hemoglobin to Oxygen
What is the net charge when finishing Stage 1 of glycolysis?
What is the net charge when finishing Stage 1 and Stage 2 only once of glycolysis?
What is the net charge when finishing Stage 1 and Stage 2 twice of glycolysis?
What energy is spent when finishing Stage 2 of glycolysis?
-2 ATP (used up 2 ATP, didn’t get anything back)
0 ATP (used up 2 ATP, got 2 ATP back in 2nd stage)
+2 ATP (used up 2 ATP, got 4 ATP back in complete 2nd stage)
2 NAD+
Once glycolysis converts glucose into pyruvate and generates ATP, what needs to happen for ATP synthesis to continue?
NADH needs to be reoxidized (reverted) to NAD+
What are three consequences of excess consumption of fructose?
What does fructose do in the liver that makes it so bad?
Fatty liver, type two diabetes, and obesity
Bypasses the key regulatory enzyme phosphofructokinase (PFK)
What is galactosemia?
What are the symptoms (2 things)?
How many states in the US screen for galactosemia in newborns?
When Galactose-1-phosphate (Gal-1-P) accumulates in the erythrocytes (RBCs) of a patient
Liver enlargement, cataract formation
All 50
What enzyme breaks down cAMP and terminates the signal of epinephrine?
Phosphodiesterase enzyme
What is the difference between hexokinase in the muscle and glucokinase in the liver?
Hexokinase is in the cytoplasm and has a higher affinity towards glucose, so it won’t allow glucose to roam freely, while glucokinase is in the nucleus and has a lower affinity towards glucose, so it allows the liver to provide glucose to the body when needed
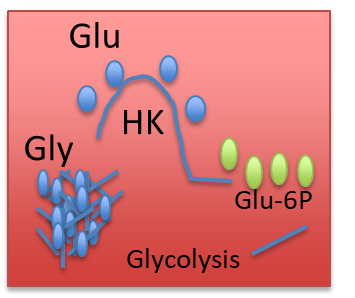
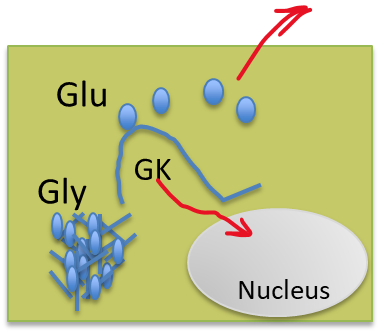
(Note: Red is hexokinase, Green is glucokinase)
What is the most potent PFK activator?
What does it do?
Fructose 2,6-bisphosphate (F-2,6-BP)
Once plenty of fructose 6-phosphate is made, it creates PFK-2 to be saved until enough glucose has been made, then activating PFK-1 to make the process of glycolysis go much faster
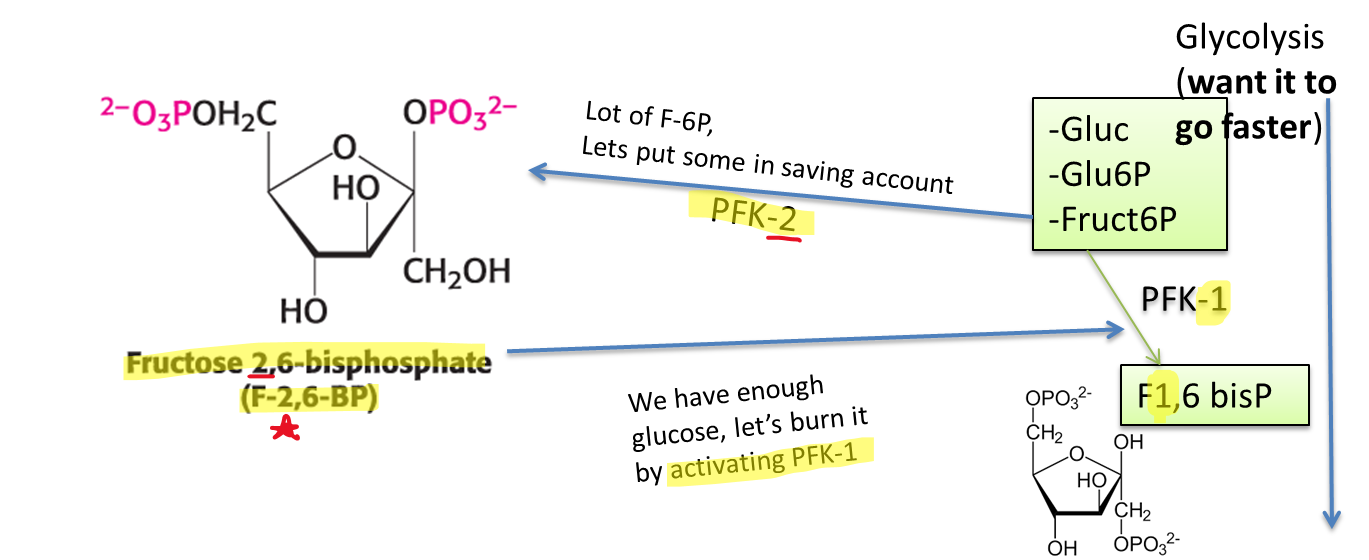
(Note: This is happening in the liver, so glucokinase is being used rather than hexokinase)
The major site of gluconeogenesis is in the…?
Liver
Where does the formation of oxaloacetate by pyruvate carboxylase take place in?
What is this used for?
Mitochondria
Gluconeogenesis in the cytoplasm
What enzyme is used to convert fructose 1,6-bisphosphate to fructose 6-phosphate?
Fructose 1,6-bisphosphatase
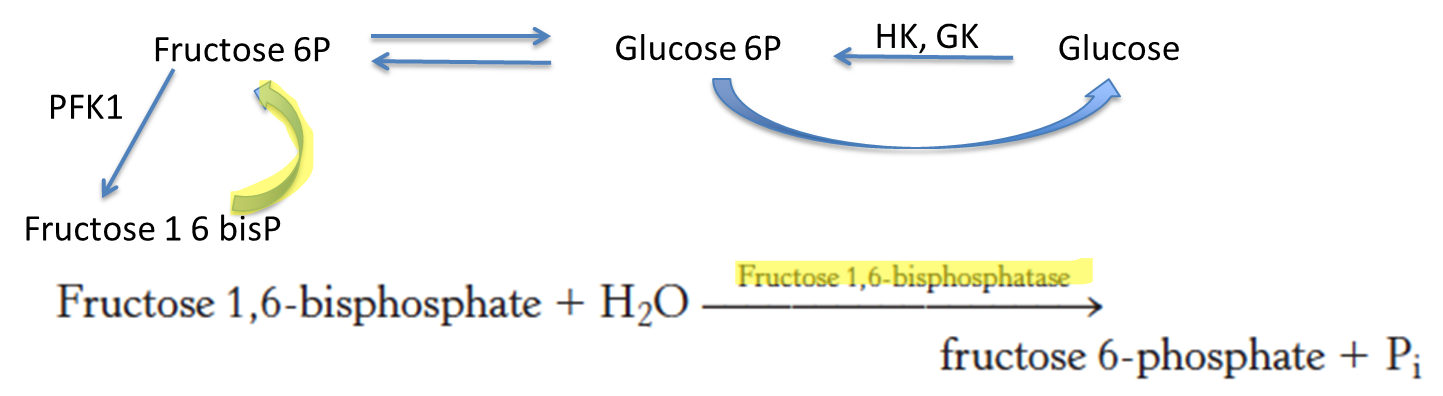
What does glucose increase the concentration of?
What is the role of this increase?
Fructose 2,6-bisphosphate (F-2,6-BP)
Increase glycolysis and inhibit glyconeogenesis
When there is less glucose in the body, what happens to PFK1 activation, PFK1 gate, and the overall result of glycolysis?
When there is lots of glucose in the body, what happens to PFK1 activation, PFK1 gate, and the overall result of glycolysis?
No PFK1 activation, PKF1 gate is closed, glycolysis slows down
(Note: Glycolysis slows down because the body wants to store what little glucose the body has)
PFK1 activation, Lots of F-2,6-BP in the reservoir, PFK1 gate opens, glycolysis speeds up
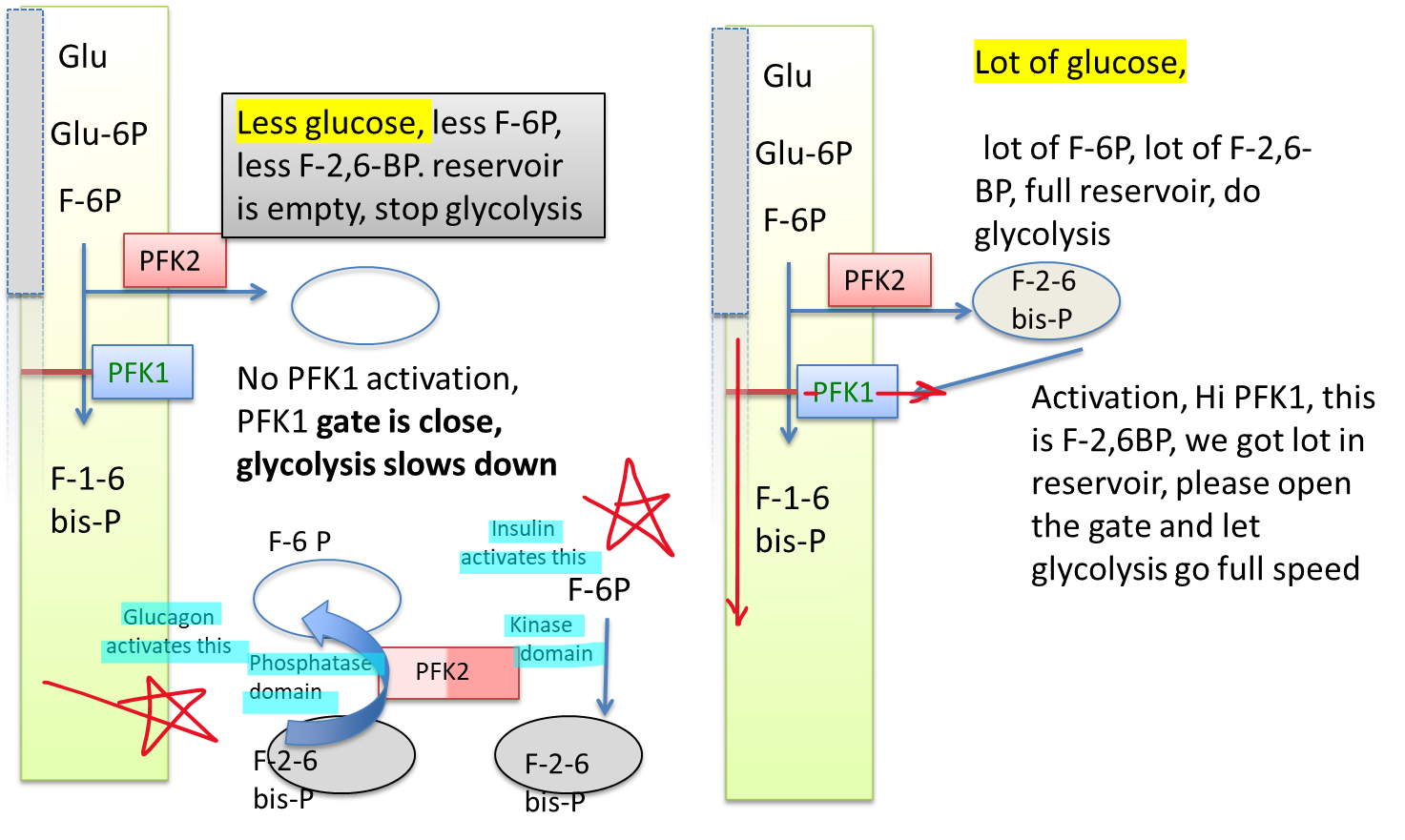
What domain is responsible for an empty reservoir? What hormone activates this?
What domain is responsible for a full reservoir? What hormone activates this?
Phosphatase domain. Glucagon
Kinase domain. Insulin
What is used and produced when converting oxaloacetate to malate? What is this reaction called?
What is used and produced when converting malate to oxaloacetate? What is this reaction called?
Use NADH, produce NAD+. Reduction reaction
Use NAD+, produce NADH. Oxidation reaction
Who is phosphorylating tyrosines (Y) of IRS-1?
Tyrosine kinase (activated insulin receptor)