LESSON #2: STATES OF MATTER RELATED TO PHARMACEUTICAL FORMULATIONS
1/136
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
Cohesive forces
Type of forces where like molecules are attracted to each other (e.g., water to water)
Adhesive forces
Type of forces where different molecules are attracted to one another (e.g., water to beaker)
Lennard-Jones Potential Graph
A model that describes that as two molecules approach each other (at a moderate distance), the energy changes are gradual and negative (attractive) to a point where a minimum in the potential energy occurs.
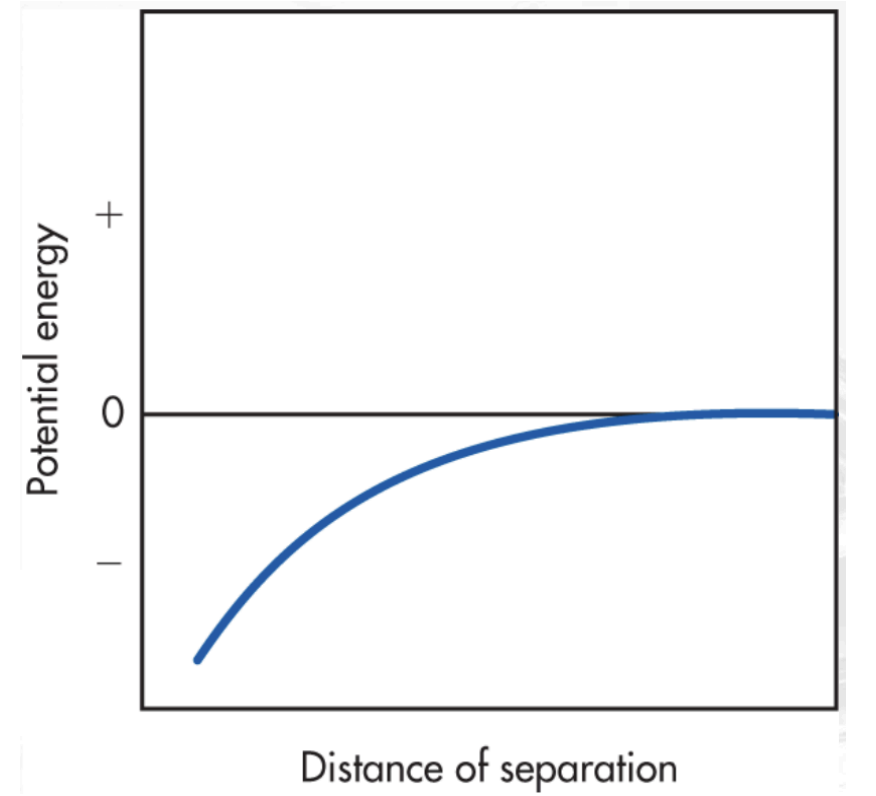
Increasing exponential graph from negative to 0
When molecules are far apart, they start to approach each other, meaning attractive forces start to form to a point where a minimum in potential energy occurs. There is an increase in negative potential energy.
Describe how this looks on a graph.
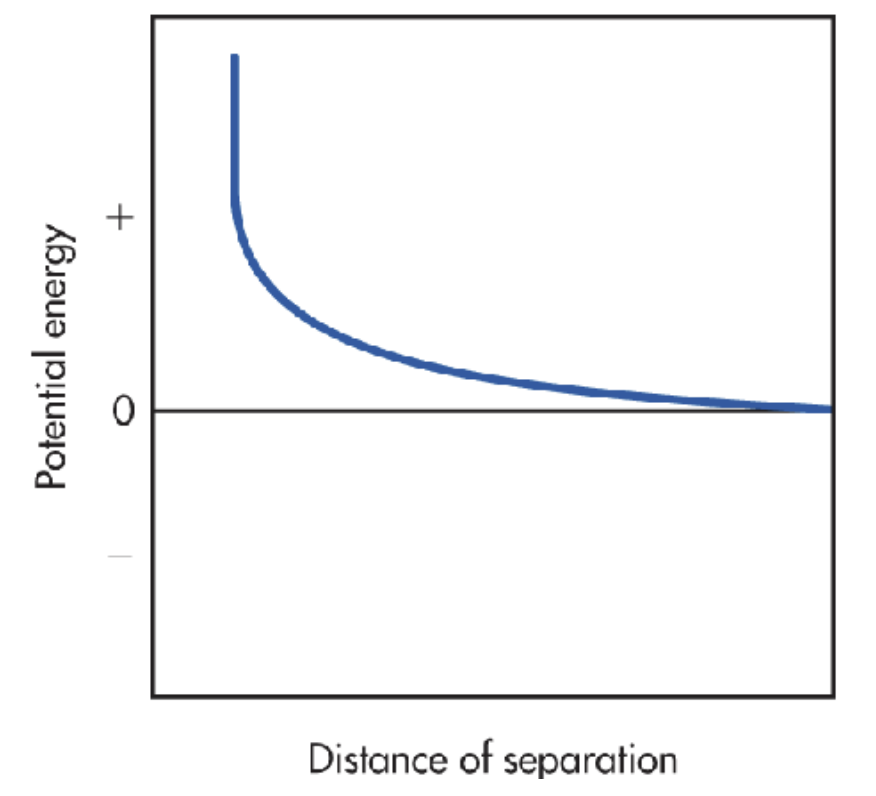
Decreasing exponential graph from positive to 0
As the energy starts rising rapidly (due to the attractive forces) as the intermolecular distances become smaller, repulsive forces begin to dominate, meaning resistance is starting to form. There is an increase in positive potential energy.
Describe how this looks on a graph.
Attraction
Repulsive
The Lennard-Jones Potential graph shows how the energy between two molecules changes as the distance between them changes. It basically says that at:
Far distance, there is _______ forces, while at
Close distance, there is _______ forces.
Potential energy
It is the energy stored in an object.
Keesom forces
Other name for Dipole-dipole forces.
Debye forces
Other name for Dipole-induced dipole forces.
London Dispersion forces
Other name for Induced dipole-induced dipole forces.
1-7 kcal/mol
Van der Waals Forces: Energy (kcal/mol) of Dipole-dipole (Keesom forces)
1-3 kcal/mol
Van der Waals Forces: Energy (kcal/mol) of Dipole-induced dipole (Debye forces)
0.5-1 kcal/mol
Van der Waals Forces: Energy (kcal/mol) of Induced dipole-induced dipole (London forces)
protein secondary structure (α–helices)
Van der Waals Forces: Dipole-dipole (Keesom forces) stabilize __________________.
effect on states of matter
Van der Waals Forces: Dipole-induced dipole (Debye forces) stabilize ________________.
liquefaction
dipole interactions
bonding
Van der Waals Forces: Induced dipole-induced dipole (London forces), causes ___________ of gases, molecular _______________ in solubility, complexation, and other physical _________ phenomena
H2O, HCl, alcohol, acetone, phenol
Examples of Dipole-dipole (Keesom forces)
ethyl acetate, methylene chloride, ether
Examples of Dipole-induced dipole (Debye forces)
1-7 kcal/mol
Ion-dipole Forces: Energy (kcal/mol) of Ion-dipole
Quaternary ammonium ion with tertiary amine
Examples of Ion-dipole
pharmaceutical salts
Ion-dipole Forces: Ion-dipole acts as ________________.
KI (enhance solubility) + iodine
Examples of Ion-induced dipole
ionic crystalline subs
Ion-induced dipole has a solubility of _________________ in H2O.
Hydrogen Bonding
Intermolecular Attractive Forces: Attraction of a hydrogen atom for a strongly negative atom
Hydrogen Bonding
Applications of _____________ protein α–helix and β–sheet structures, conformation of proteins, physical properties of alcohols compared to alkanes; carboxylic acids compared to esters, aldehydes, and ketones; sugars.
Hydrophobic interaction
Intermolecular Attractive Forces: forces of attraction between nonpolar atoms and molecules in water
Hydrophobic interaction
Applications of _______________ are its structure stabilizes molecules including proteins and bilayer membrane structures.
Gas
States of Matter:
no regular shape; capable of filling available space
higher kinetic energy; weak intermolecular forces
compressible
Liquid
States of Matter:
occupies a definite volume; takes the shape of the container
denser and possesses less kinetic energy than gases
less compressible than gases more compressible than solids
Solid
States of Matter:
Have a fixed shape
strong intermolecular forces, little kinetic energy
nearly incompressible compared to other states
Ideal Gas Law
Gas: Equational law that is useful in calculating properties of gases at atmospheric pressure and at temperatures above their boiling points.
PV = nRT
Gas: The ideal gas law equation has variables that are directly proportional to each other. What is the equation?
Boyle’s Law
Gas: States that volume is inversely proportional to pressure.
Charles’ Law
Gas: States that volume is directly proportional to temperature.
Avogadro’s Law
Gas: States that volume is directly proportional to the number of moles
Partial Pressure
Gas: The higher the concentration, the higher the __________________.
Henry’s Law of Gas Solubility
Gas (Blood gases, oxygen and carbon dioxide): Law that states that the amount of gas dissolved in the plasma is proportional to the partial pressure of the gas in equilibrium with the plasma
Dalton’s Law of Partial Pressures
Gas (Blood gases, oxygen and carbon dioxide): Law that states that the partial pressure is the pressure a gas would exert if it alone occupied the whole volume of the mixture
Vapor Pressure
Liquids: pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase in a closed system at a given temperature.
directly
Liquids (Vapor Pressure): Vapor pressure and temperature are ____________ proportional.
inversely
Liquids (Vapor Pressure): Vapor pressure and boiling point are _____________ proportional.
inversely
Liquids (Surface Tension): Surface tension and temperature are ______________ proportional.
Surface Tension
Liquids: the measure of force per unit length.
crystalline, polymeric, and polymorphs
Solids: The three types of solids are __________, __________, and __________.
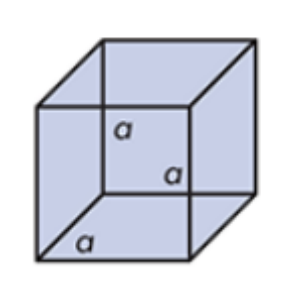
Cubic
Sodium chloride
What do you call this crystalline solid? Give an example.
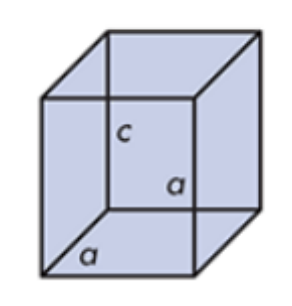
Tetragonal
Urea
What do you call this crystalline solid? Give an example.
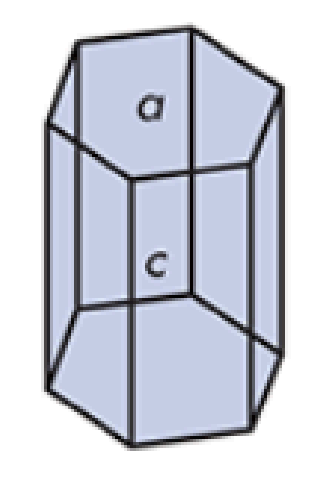
Orthorhombic
ritonavir Form II
What do you call this crystalline solid? Give an example.
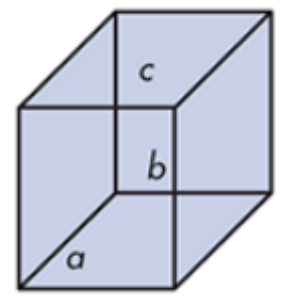
Rhombohedral
iodine
What do you call this crystalline solid? Give an example.
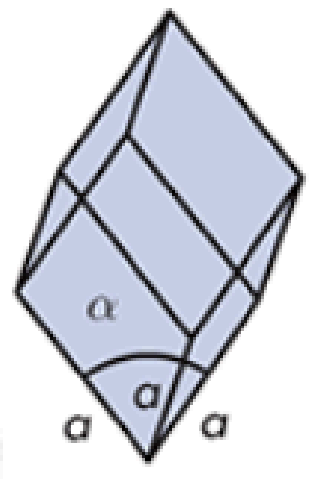
Hexagonal
iodoform
What do you call this crystalline solid? Give an example.
Monocyclic
sucrose, and ritonavir Form 1
What do you call this crystalline solid? Give an example.
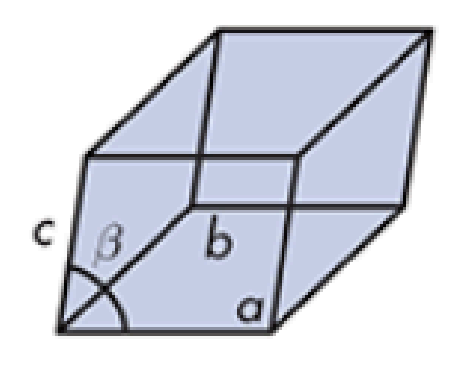
Triclinic
Boric acid
What do you call this crystalline solid? Give an example.
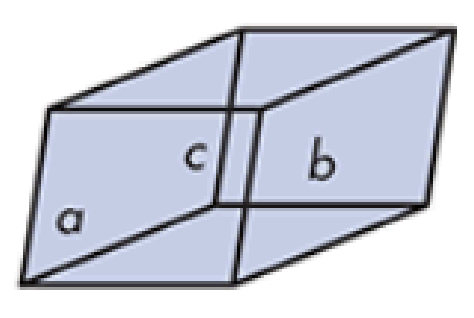
three-dimensional lattice
Solids: Molecules or atoms are arranged in repetitious _______________ units (unit cell) infinitely throughout the crystal
Atom to atom
Type of Crystal Bonding described below:
Example: Carbon, diamond
Bonding: Strong carbon covalent bonds
Physical Characteristics: Hard, large crystals
Metallic
Type of Crystal Bonding described below:
Example: Silver
Bonding: Strong metal bond
Physical Characteristics: Positive ions in a field of freely moving electrons
Molecular
Type of Crystal Bonding described below:
Example: Menthol, paraffin
Bonding: Van der Waals forces
Physical Characteristics: Close packing, weakly held together, low melting point
Ionic
Type of Crystal Bonding described below:
Example: NaCl
Bonding: Electrostatic ionic bond
Physical Characteristics: Hard, close packing, strongly held together, high melting point
Polymorphs
Solids: type of solid structure that may exist in more than one crystalline structure
intermolecular bonding patterns, conformational changes, and molecular orientations
Changes in Crystalline Forms include _________________, _____________, and ________________.
Melting point
Solubility
Stability
Physical properties included in polymorphs.
3
Polymorphic (# of Forms): Acetaminophen
2
Polymorphic (# of Forms): Caffeine
2
Polymorphic (# of Forms): Phenobarbital sodium
2
Polymorphic (# of Forms): Phenytoin
4
Polymorphic (# of Forms): Chloramphenicol palmitate
3
Polymorphic (# of Forms): Cimetidine
2
Polymorphic (# of Forms): Nifedipine
2
Polymorphic (# of Forms): Progesterone
2
Polymorphic (# of Forms): Theophylline
Hydrate
Solvates and Hydrates: Form where water is included in a lattice, commonly USED as a drug substance. It is less soluble in water or aqueous mixtures than anhydrous forms
Solvate
Solvates and Hydrates: Form that is incorporated into the lattice. It is NOT chosen as drug substances due to possible toxicity of common solvents.
Salt crystals
Solvates and Hydrates: A lattice accommodating other molecules to form salts. It is when two (2) ionized compounds will interact with the lattice to form a crystalline salt.
Cation/Counterion
Salt crystals: the corresponding compound in a salt. Its properties include melting point, stability, solubility, dissolution, bioavailability.
53.4 %
Common Counterions (Anion): Frequency of Hydrochloride
7.5%
Common Counterions (Anion): Frequency of Sulfate
4.6%
Common Counterions (Anion): Frequency of Bromide
4.2%
Common Counterions (Anion): Frequency of Maleate
4.2%
Common Counterions (Anion): Frequency of Mesylate
3.8%
Common Counterions (Anion): Frequency of Tartrate
3.3%
Common Counterions (Anion): Frequency of Acetate
2.7%
Common Counterions (Anion): Frequency of Citrate
2.7%
Common Counterions (Anion): Frequency of Phosphate
75.3%
Common Counterions (Cation): Frequency of Sodium
6.9%
Common Counterions (Cation): Frequency of Calcium
6.3%
Common Counterions (Cation): Frequency of Potassium
2.9%
Common Counterions (Cation): Frequency of Meglumine
1.7%
Common Counterions (Cation): Frequency of Tromethamine
1.2%
Common Counterions (Cation): Frequency of Magnesium
1.2%
Common Counterions (Cation): Frequency of Zinc
0.6%
Common Counterions (Cation): Frequency of Lysine
0.6%
Common Counterions (Cation): Frequency of Diethylamine
Cocrystals
Homogeneous multicomponent phase of fixed stoichiometry where the chemical entities are held together in a crystal lattice by intermolecular forces.
water and solvents
Cocrystals also contain _______ and ______ to form cocrystalline hydrates
ionizable group
Cocrystals are a good option to change properties when an ___________ is not available
Amorphous solids
Solids: No long-range order over many molecular units to produce a lattice or crystalline structure
Glasses: nonequilibrium solid form
Supercooled liquids: viscous equilibrium liquid form
Amorphous solids:
Glasses: _________________ form
Supercooled liquids: ________________ form
Do not
Amorphous solids do or do not possess melting points?
Glass transition (Tg) temperature
temperature where an amorphous material converts from a glass to a supercooled liquid upon heating
less
more
Amorphous solids are _____ physically stable but _____ soluble than crystalline materials
surfactants
An amorphous drug is stabilized by a polymer or a combination of polymers or ___________
Surfactants
Also referred to as surface-active agents. When they are placed in a solution, they will act at the boundary of two materials or two states of matter