MD4: Steroids
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Where are corticosteroids found
Adrenal cortex
Secreted following signalling from hypothalamus in hypothalamic pituitary adrenal axis
mainly hydrocortisone aka cortisol
what is the effect of glucocorticoid
Reduced inflammation
Reduced immune response
Redistributes fat
Hyperglycaemia
What is the effect of mineralcorticoid
Reabsorption of na+
Secretion of K+
Secretion of H+
Increases BP and blood volume
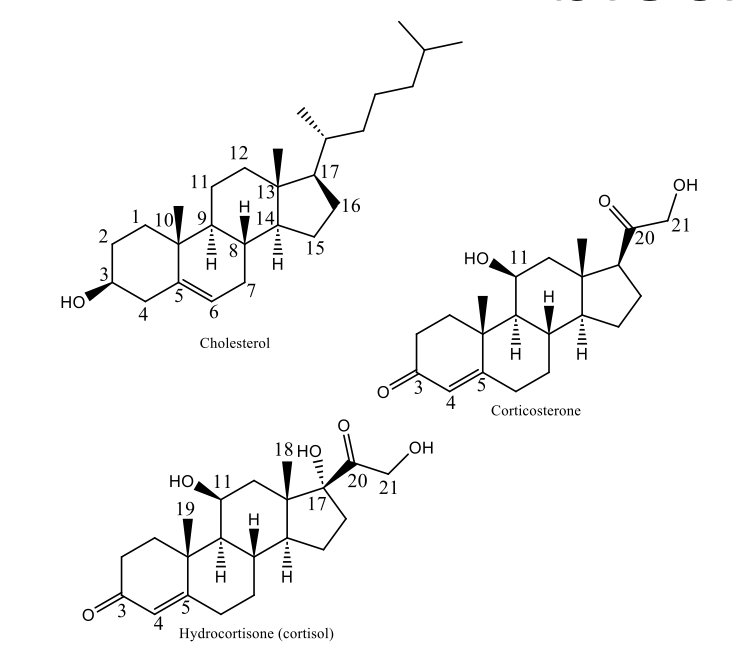
Structure of hydrocortisone and corticosterone
Conjugated Ketone at C3
beta OH, 2 carbons away from carbonyl
17 beta ketol side chain
What is the ideal properties of inflammatory diseases
High potency
High selectivity for glucocorticoid activity
Low sodium retaining potency
Increased affinity for glucocorticoid receptors to use lower doses and reduce side effects
What is the essential functional group for steroid anti-inflammatory activity?
An oxygen at the 11-position, which can be in the form of a ketone (11-oxo) or an alcohol (11-beta-hydroxy).
What are key structural modifications that increase glucocorticoid selectivity and potency?
Flattening the A-ring by introducing a 1,2 double bond (sp³ → sp²). more stable, more electronegative
Adding an electron-withdrawing halogen at C-9. This increases the acidity, more readily available to donate electrons more potent
Esterification at C-17 or cyclic acetals at C-16,17. increases potency and lipophilicity
Adding a C-16 methyl group (increases selectivity without affecting potency).
How does flattening the A-ring improve glucocorticoid potency?
A 1,2 double bond (sp³ → sp²) increases receptor binding, making prednisolone more potent than hydrocortisone.
Why is prednisolone preferred over hydrocortisone for oral treatment?
Prednisolone has higher potency and fewer side effects compared to oral hydrocortisone.
It is best given in the morning to mimic the endogenous release of cortisol
What is the mechanism of beclomethasone ester hydrolysis?
Beclometasone 17,21-dipropionate is hydrolyzed in the lung by esterases to beclometasone 17-monopropionate and then to active beclometasone.
prodrug
diester = lipophilic = increased absorption
Why is hydrocortisone 17-butyrate much more potent than hydrocortisone?
Esterification at the 17-position increases both potency and lipophilicity, leading to better absorption and prolonged action.
It is also slowly and partially hydrolysed
Why is clobetasol much more potent than clobetasone?
Clobetasol has structural modifications that enhance glucocorticoid receptor affinity, increasing its potency as a topical steroid.
longer carbon chains are more lipophilic
Longer hydrocarbon chains decrease molecules solubility
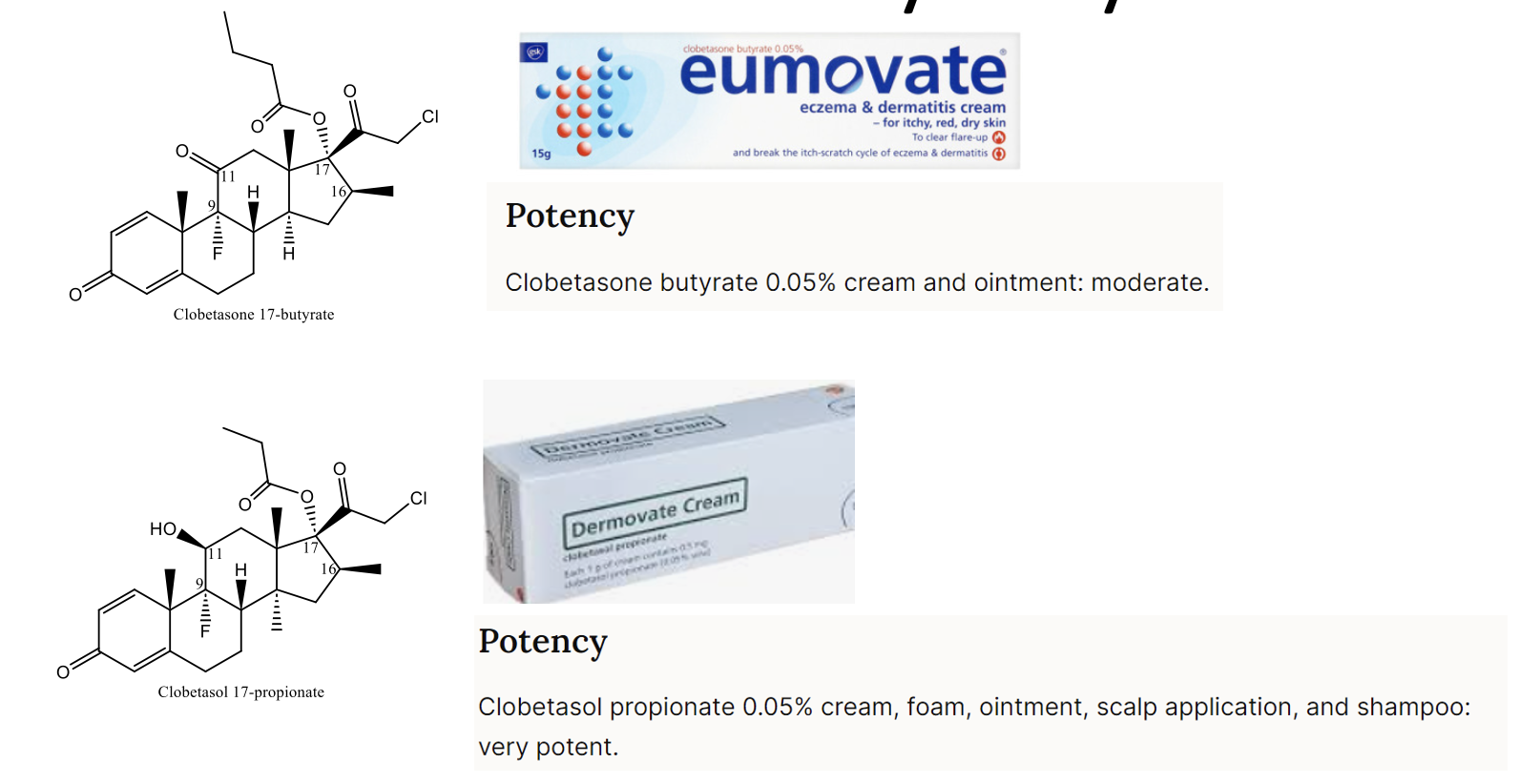
What are the potencies of topical steroids covered in the lecture?
Mild: Hydrocortisone (up to 2.5% w/w) - Moderate: Clobetasone butyrate (0.05% w/w) - Potent: Hydrocortisone butyrate (0.1% w/w) - Very Potent: Clobetasol propionate (0.05% w/w)
How should steroid potency match eczema severity?
Mild eczema → Mild steroids - Moderate eczema → Moderately potent steroids - Severe eczema → Potent/very potent steroids
Why do oral steroids require a significantly different dose than topical/inhaled steroids?
Due to first-pass metabolism, systemic distribution, and differences in local drug concentration at the site of action.
How can lipophilicity be increased in topical corticosteroids?
By converting hydroxyl (-OH) groups into esters, which enhances absorption and potency (optimal LogP < 5).
What is the hypothalamic-pituitary-adrenal (HPA) axis?
A communication system between the hypothalamus, pituitary gland, and adrenal glands that regulates corticosteroid production.
What is the difference between glucocorticoid and mineralocorticoid activity?
Glucocorticoid activity → Reduces inflammation (desired in steroid drugs). - Mineralocorticoid activity → Affects electrolyte balance (unwanted side effects).