1. CNS 5 & 6 Neurotransmitters & Neuromodulators
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
What is the difference between a neurotransmitter & a neuromodulator?
Neurotransmitter = endogenous chemical that transmits signals across a synapse to another neurone, muscle, or effector cell
Neuromodulator = endogenous chemical affecting groups of neurones or cells with the right receptor, often not released at synapses
Acts via second messengers
Produces longer-lasting effects
Endogenous = made naturally within the body
What are the major types of neurotransmitters & neuromodulators?
Acetylcholine – NT
Monoamines – NT & NM
Catecholamines: dopamine, noradrenaline, adrenaline
Indolamines: serotonin
Amino acids – NT & NM
Glutamate, GABA, glycine
Peptides – NT & NM
Endorphins, enkephalins
Lipid-like substances – NT
Anandamide, leptin
Nucleosides – NM
Adenosine
Soluble gases – NM
Nitric oxide, carbon monoxide
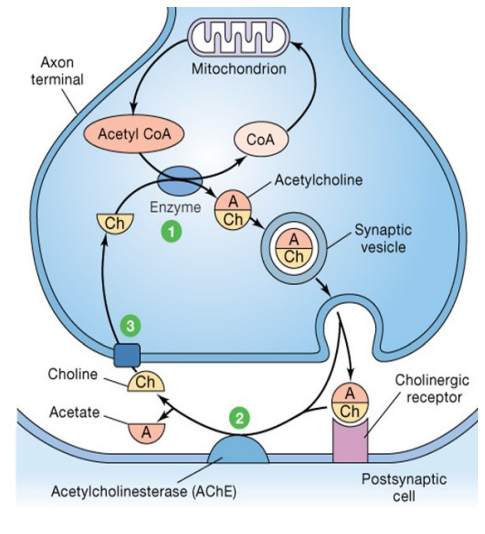
What are the key features of acetylcholine (ACh) as a neurotransmitter?
Found in PNS – causes skeletal muscle contraction
In ANS & brain – involved in plasticity, arousal & reward
Synthesised from choline + acetyl-CoA in presynaptic terminals
Stored in vesicles & released via exocytosis
Binds to cholinergic receptors = excitatory or inhibitory effects
Removed by acetylcholinesterase (AChE)
Choline is reuptaken by a specific transporter
What are the major acetylcholine pathways in the brain & their functions?
Nucleus Basalis of Meynert
Neocortex = memory (via M1 receptors)
Frontal cortex = memory (via M1 receptors)
Hippocampus = spatial memory
Amygdala = emotions
Dorsolateral Tegmental Nucleus
Cerebellum = balance & movement
Thalamus = sensory processing
Striatum (basal ganglia) = motor control via cholinergic interneurons
ACh neurons are localised but project widely to modulate multiple brain functions
What are the types of acetylcholine (ACh) receptors, their agonists & antagonists?
Nicotinic ACh receptors
Stimulated by: nicotine
Blocked by: curare
Muscarinic ACh receptors
Stimulated by: muscarine
Blocked by: atropine
ACh receptors are widely distributed & regulate many central & peripheral functions
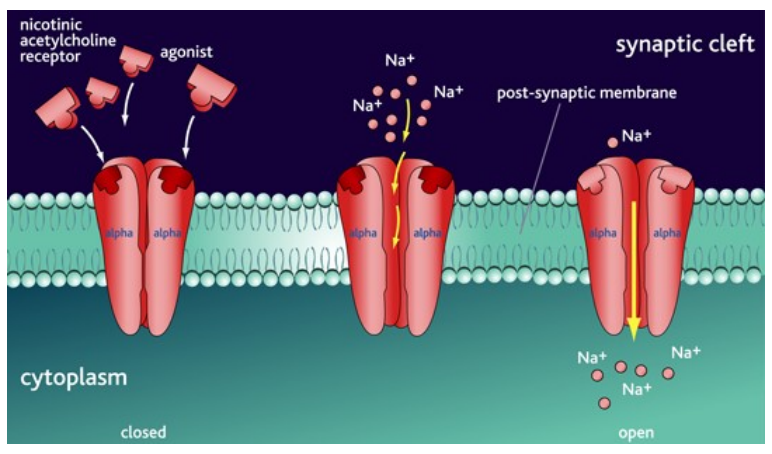
What are the key features of nicotinic ACh receptors in the brain?
Ligand-gated ion channels on neurones
Activated by ACh = Na⁺ influx
Causes depolarisation (EPSP) = ↑ excitability
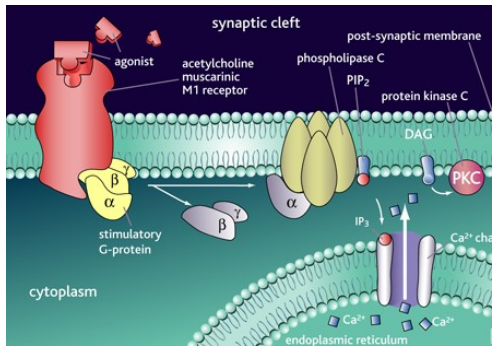
What are the roles and signaling mechanisms of muscarinic ACh receptors?
M1, M3, M5:
Activate Gq/11 proteins = excitation
Phospholipase activation = ↑ intracellular Ca²+ = depolarization
Slow EPSP, regulate K+ channels
M2, M4:
Activate Gi/O proteins = inhibition
Inhibit adenylyl cyclase = ↓ cAMP
↓ Protein kinase A activity = reduced phosphorylation of channels
Inhibit voltage-gated Ca²⁺ channels
Effect: Dependent on receptor type and brain location, leading to either excitatory or inhibitory responses.
What are the types and examples of ACh receptor agonists and antagonists?
Acetylcholinesterase inhibitors:
Reversible: e.g., neostigmine (treatment for Myasthenia gravis)
Irreversible: e.g., parathion (chemical weapon)
Nicotinic receptor agonists:
Stimulate memory (e.g., nicotine)
Drug candidates for Alzheimer's disease, schizophrenia, ADHD (e.g., Galantamine)
Nicotinic receptor antagonists:
Mild sedative & cough suppressant (e.g., DXM)
Muscarinic receptor agonists:
Treatments for Alzheimer's disease (e.g., M1 receptor agonists)
Treatment for glaucoma (e.g., pilocarpine, M3 receptor agonists)
Muscarinic receptor antagonists:
Atropine & scopolamine (toxins)
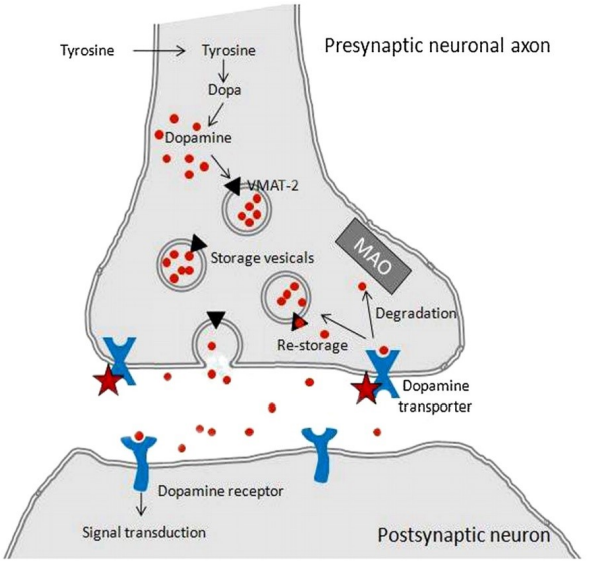
What are the types & key features of dopamine as a neurotransmitter?
Type: Catecholamine
Synthesised from: Tyrosine in dopaminergic neurones
Stored in: Large dense core vesicles
Released by: Vesicle exocytosis (AP-triggered)
Receptor action: Binds dopamine receptors → excitatory or inhibitory (receptor-dependent)
Removed by: Dopamine transporters (DAT)
Degraded by: Monoamine oxidase (MAO)
Functions:
Movement control (via basal ganglia)
Emotional response (via limbic system)
Pleasure & pain control (via nucleus accumbens)
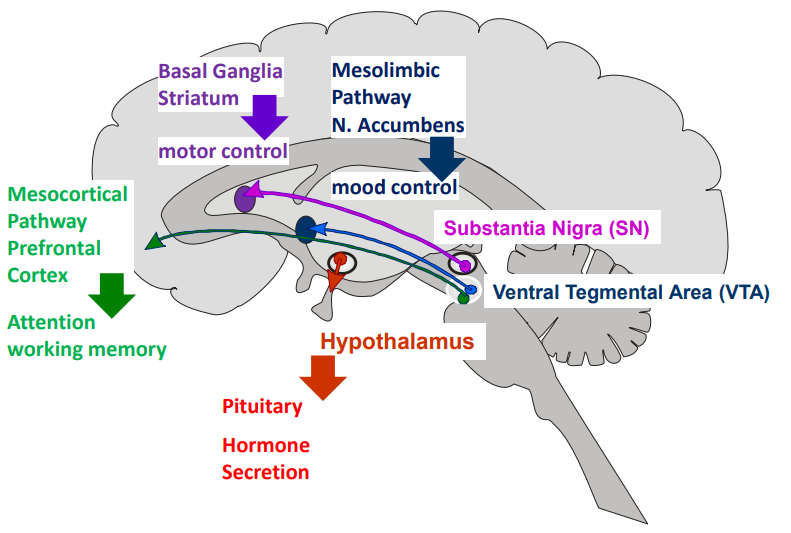
What are the major dopamine pathways in the brain & their functions?
Nigrostriatal (Substantia Nigra → Striatum):
Motor control (↓ dopamine here = Parkinson’s motor symptoms)
Mesolimbic (VTA → Nucleus Accumbens):
Mood, reward, addiction
Mesocortical (VTA → Prefrontal Cortex):
Attention, working memory, cognition
Tuberoinfundibular (Hypothalamus → Pituitary):
Regulates hormone secretion
What are the mechanisms of D₁-like & D₂-like dopamine receptors?
D₁-like receptors (D₁, D₅)
• Coupled to Gₛ = ↑ adenylyl cyclase = ↑ cAMP = ↑ PKA = excitatoryD₂-like receptors (D₂, D₃, D₄)
• Coupled to Gᵢ/ₒ = ↓ adenylyl cyclase = ↓ cAMP = ↓ PKA = inhibitoryBalance is key in regions like basal ganglia
• Imbalance = motor disorders (e.g. Parkinson’s)
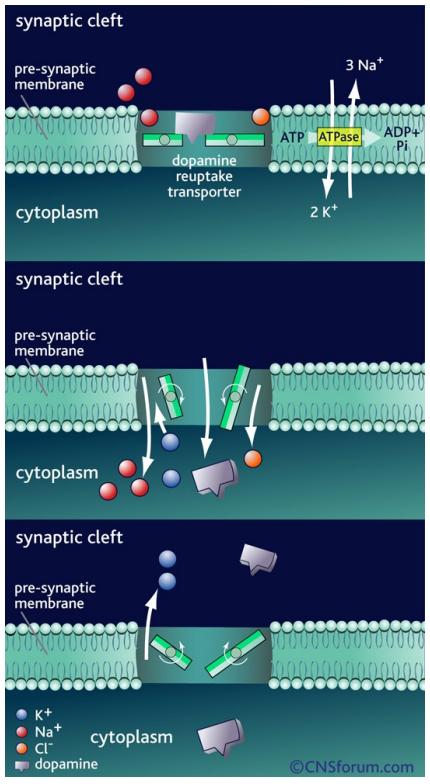
How is dopamine action terminated at the synapse?
Re-uptake into presynaptic neuron via dopamine transporter (DAT)
Driven by Na⁺/K⁺ gradients maintained by Na⁺/K⁺-ATPase (via ATP hydrolysis)
Co-transport of dopamine with Na⁺ & Cl⁻ into the neuron
K⁺ binding resets transporter to outward position; release into synaptic cleft restores ionic gradient
What are the roles of dopamine agonists & antagonists?
Agonists/mimetics:
DA transporter inhibitors (↑ synaptic DA) — e.g. amphetamine, cocaine
DA precursor (↑ DA synthesis) — L-DOPA for Parkinson’s
Antagonists:
D₂ antagonists — typical antipsychotics for schizophrenia
Can cause Parkinson-like symptoms
↑ prolactin release
Tranquillizing/sedative effects
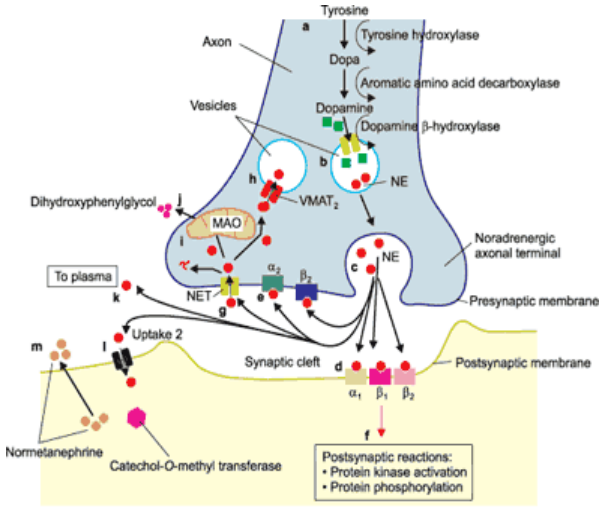
What is the role of noradrenaline (NA) in the brain and body, and how is it synthesized, released, and removed from the synaptic cleft?
Neurotransmitter of the catecholamine family
Roles: autonomic control (sympathetic nervous system), alertness, rest cycles, attention, & memory in the CNS
Synthesized from tyrosine in presynaptic terminals of adrenergic neurones
Stored in large dense-core vesicles
Released by vesicle exocytosis
Binds to adrenergic receptors, causing excitatory or inhibitory postsynaptic effects
Removed from synaptic cleft by adrenergic transporters
Degraded by monoamine oxidase (MAO) & catechol-O-methyltransferase (COMT)
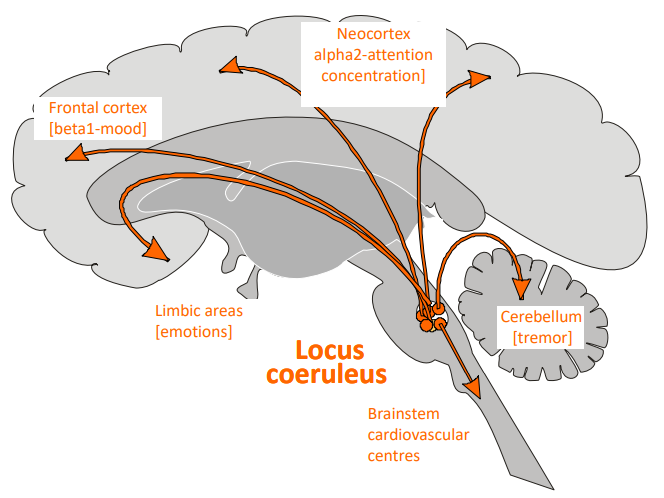
Where are the main pathways of noradrenaline (NA) in the brain, and what functions do they regulate in different regions?
Locus coeruleus: Cell bodies of adrenergic neurones located in the pons; axons project to multiple brain areas.
Neocortex (α2 receptors): Regulates attention, concentration, and cognitive function.
Frontal cortex (β1 receptors): Regulates mood.
Limbic areas: Regulates emotions.
Cerebellum: Regulates tremor and movement coordination.
Brainstem (Cardiovascular centres): Regulates cardiovascular functions.
Associated neurological disorders:
Affective disorders
Depression
Autonomic failure
Pain
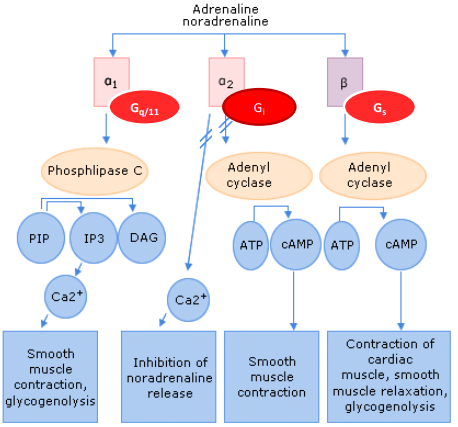
How does noradrenaline exert its effects on the brain, and how do the different receptors (α1, α2, β) contribute to excitatory and inhibitory functions?
Receptors: Noradrenaline acts on different receptors in the brain, similar to peripheral receptors.
α1 receptors: Coupled to the phospholipid pathway via Gq proteins, leading to excitatory effects.
α2 receptors: Coupled to Gi proteins, inhibiting adenylate cyclase, leading to inhibitory effects.
β receptors: Coupled to Gs proteins, stimulating adenylate cyclase, resulting in excitatory effects.
Neuronal activity: These receptors are expressed by neurons in the brain and regulate various functions depending on the type of receptor activated.
Excitatory and inhibitory effects:
α1 and β receptors are excitatory.
α2 receptors are inhibitory.
How is noradrenaline re-uptake terminated at the synapse?
Re-uptake occurs via an energy-dependent process across the presynaptic membrane.
Sodium/potassium ATPases use ATP hydrolysis to create an ion gradient.
This gradient drives transporter opening, co-transporting Na+, Cl-, and DA.
Potassium ions bind to the transporter, enabling it to return to the outward position.
What are the agonists and antagonists of noradrenaline?
Agonists & Mimetics:
NA transporter inhibitors: amphetamine, cocaine (increase wakefulness, alertness, reduce fatigue; drugs of abuse)
Tricyclic antidepressants: imipramine (uptake inhibitors for NA & 5-HT)
MAO inhibitors: impair cognitive processes, cause euphoria, insomnia, hallucinations, delusions
Antagonists:
Beta blockers: anxiolytic effects
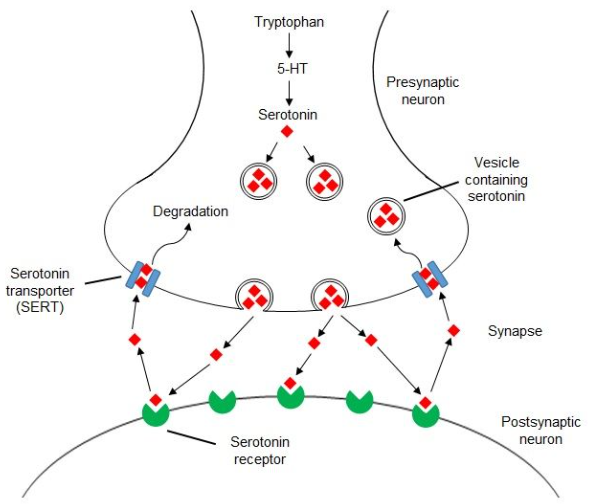
What is serotonin and its role in the brain and body?
Serotonin (5-HT): A neurotransmitter of the indolamine category.
Functions:
Controls mood, appetite, sleep
Regulates cognitive functions like memory and learning
Synthesis: Made in presynaptic terminals from tryptophan, stored in large dense core vesicles.
Release: Released via exocytosis from vesicles.
Receptors: Binds to 5-HT receptors, producing excitatory or inhibitory effects.
Termination: Removed from the synaptic cleft by specific transporters and degraded by monoamine oxidase (MAO).
What are the serotonin pathways in the brain and their functions?
Raphe Nucleus: Located at the border between the pons and midbrain, it is the source of serotonergic neurons.
Frontal Cortex: Mood regulation.
Limbic Areas: Involved in anxiety and panic.
Basal Ganglia: Regulates movement, obsessions, and compulsions.
Spinal Cord: Regulates nociception and sexual dysfunction.
Hypothalamus: Involved in appetite regulation.
Sleep Centers: Found in the reticular formation, involved in vomiting.
Associated Disorders: Depression, anxiety, migraine.
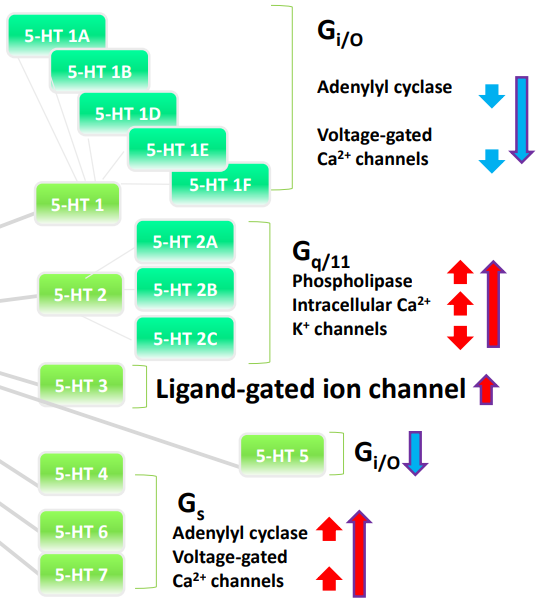
What are the effects of different serotonin (5-HT) receptor families in the brain?
5-HT1 & 5-HT5 receptors: Coupled to Gi/o proteins, leading to inhibition and a decrease in excitation.
5-HT2 family receptors: Coupled to Gq proteins, leading to excitation.
5-HT3 receptors: Ligand-gated ion channels, leading to depolarization.
5-HT4, 6, 7 receptors: Coupled to Gs proteins, leading to excitation.
How is serotonin re-uptake terminated at the synapse?
The action of serotonin is terminated by re-uptake across the presynaptic membrane, similar to dopamine and noradrenaline.
This is an energy-dependent process.
Sodium/potassium ATPases use ATP to create a concentration gradient of ions across the presynaptic membrane.
The gradient drives the transporter to co-transport Na+, Cl-, and DA from the synaptic cleft.
K+ binding to the transporter allows it to return to the outward position.
The release of K+ ions into the synaptic cleft equilibrates the ionic gradient across the presynaptic membrane.
What are some drugs that affect serotonin reuptake and serotonergic transmission?
Fluoxetine (Prozac) inhibits serotonin reuptake and is used to treat:
Depression
Some anxiety disorders
Obsessive-compulsive disorder
Hallucinogenic drugs, like LSD, interact with serotonergic transmission:
LSD stimulates 5-HT2A receptors in the forebrain, producing visual perception distortions.
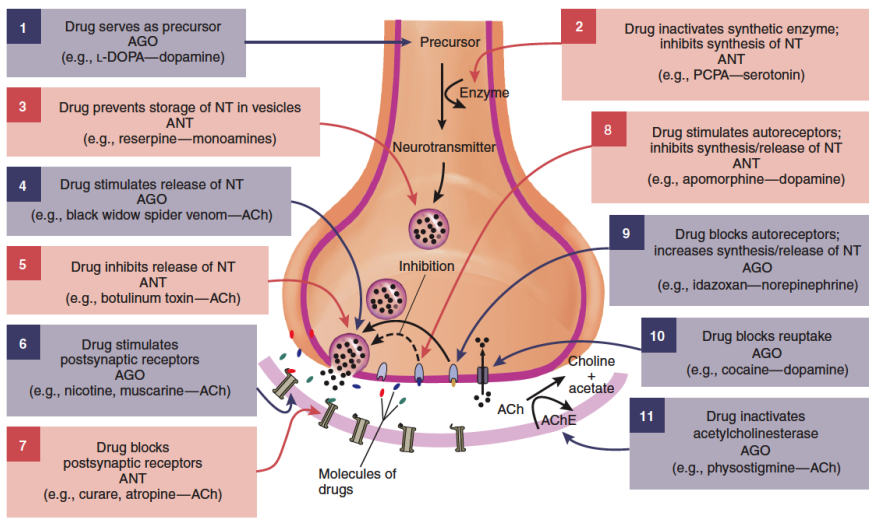
Neurotransmitter precursors: Drugs can increase (e.g., L-dopa for dopamine) or inhibit synthesis.
Neurotransmitter storage: Drugs can inhibit vesicle storage, disrupting release.
Neurotransmitter release: Drugs can stimulate or block release, but both are toxic (e.g., black widow spider venom).
Receptors:
Serotonin receptors: Specific subtypes are good targets.
GABA receptors: Multiple subtypes offer specific drug targeting.
Nicotinic receptors: Affects both CNS and periphery.
Post-synaptic & auto-receptors: Can inhibit or enhance neurotransmitter release.
Reuptake mechanisms:
Fluoxetine (Prozac) blocks serotonin reuptake.
Cocaine inhibits dopamine, serotonin, and norepinephrine reuptake.
Acetylcholinesterase inhibition: Specific to acetylcholine transmission.
What are the main challenges in CNS drug development?
Limited understanding of CNS diseases & brain function → hinders rational drug design
Drug specificity is difficult = ↑ risk of CNS side effects
Blood-brain barrier blocks most drugs = many fail to reach brain cells