MC test: S2.1 The ionic model
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Which compound will have the lowest melting point?
A. MgO
B. Al2O3
C. P4O10
D. Na2O
c. P4O10
Na2O, MgO and Al2O3 are all ionic, P4O10 is covalent.
(The actual values for Na2O, MgO and Al2O3 are 1132, 2852 and 2072 oC respectively. P4O10 melts at 340 oC.)
covalent bonds have the weakest force of attraction
→ less energy is required to break the force of bonding
Which is the correct formula for aluminium nitride?
A. Al2(NO3)3
B. Al(NO3)3
C. Al2N3
D. AlN
D. AlN
Al forms the Al3+ ion and N forms the N3− ion. NO3− is the nitrate ion not the nitride ion.
Which substance will not conduct electricity in its molten state?
A. sodium chloride
B. magnesium iodide
C. lead bromide
D. carbon tetrachloride
D. carbon tetrachloride
Carbon tetrachloride is a covalent liquid and contains no ions, all of the other compounds are ionic.
→ molten state = LOWKEY liquid
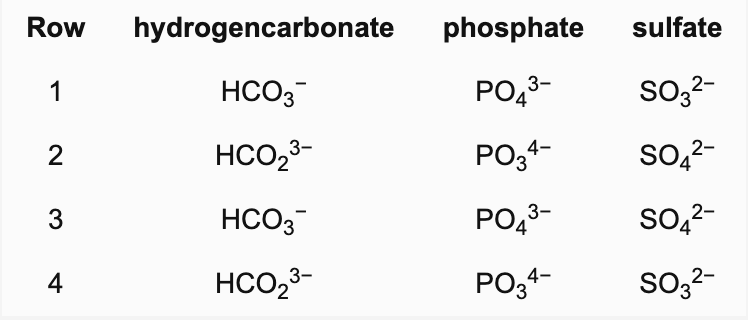
Which row contains the correct formulas for all the respective ions?
A. 2
B. 3
C. 4
D. 1
B. 3
Hydrogencarbonate: HCO3−, phosphate: PO43− and sulfate: SO42−. SO32− is the sulfite ion (sometimes called the sulfate(IV) ion).
Gadolinium is a lanthanoid. The formula of gadolinium oxide is Gd2O3. What will be the formula of gadolinium hydroxide assuming the oxidation state of gadolinium does not change?
A. Gd2(OH)3
B. Gd(OH)3
C. Gd(OH)2
D. GdOH
B. Gd(OH)3
From the formula for the oxide, gadolinium must be bonding as the Gd3+ ion. Since the hydroxide ion is OH−, the formula must be Gd(OH)3
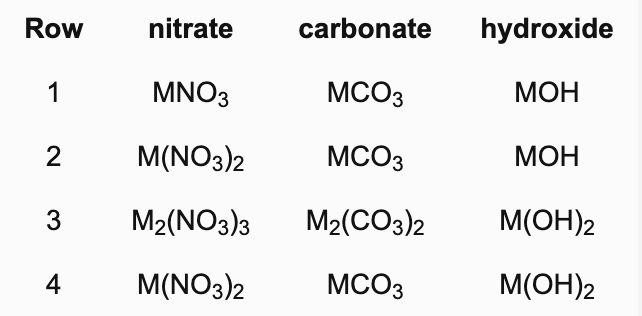
A metal M forms a nitrate, carbonate and hydroxide and has the same oxidation state in each compound. Which row contains all three correct formulas?
A. 1
B. 4
C. 2
D. 3
B. 4
The oxidation states of M are 1,2,1 respectively in row 1; 2,2,1 in row 2 and 2,2,2 in row 4. Row 3 contains the nonsensical formula M2(NO3)3 and M2(CO3)2 can be shortened to MCO3.
Which are correct statements about sodium chloride?
I. In the crystal lattice each sodium ion has six chloride ions as its nearest neighbours.
II. It can be formed by burning sodium in chlorine gas.
III. During its formation a sodium atom loses an electron and a chlorine atom gains an electron.
A. II and III only
B. I, II and III
C. I and III only
D. I and II only
B. I, II and III
Na+Cl−, which can be formed by burning sodium in chlorine ( 2Na(s) + Cl2(g) → 2NaCl(s) ), forms a face-centred cubic lattice in which each Na+ ion is surrounded by six Cl− ions and each Cl− ion is surrounded by six Na+ ions.
Which are the correct formulas for both ammonium phosphate and ammonium sulfate?
A. (NH3)2(PO4)3 and (NH3)2SO4
B. (NH4)2(PO4)3 and (NH4)2SO4
C. (NH4)3PO4 and NH4SO4
D. (NH4)3PO4 and (NH4)2SO4
D. (NH4)3PO4 and (NH4)2SO4
Ammonium is NH4+, phosphate is PO43− and sulfate is SO42− so (NH4)3PO4 and (NH4)2SO4 are correct.
Which compound contains only ionic bonding?
A. aluminium nitrate
B. sodium bromide
C. potassium carbonate
D. ammonium chloride
B. sodium bromide
Although all four are ionic compounds the nitrate and carbonate negative ions (anions) also contain covalent bonds and the ammonium positive ion (cation) contains covalent bonds between the nitrogen and hydrogen atoms.
Which will conduct electricity?
A. salt solution
B. sugar solution
C. alcohol
D. NaBr(s)
A. salt solution
Sugar solution and alcohol do not contain ions. Sodium bromide is ionic but in the solid state the ions are not free to move. Na+ and Cl− are free to move to electrodes in salt solution and chemical decomposition occurs.