Orgo Lab CA 2
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
The main separation principle in the thin-layer chromatography technique is through which of the following?
Absorption
Partition
Precipitation
Adsorption
Ion-exchange
Adsorption
After performing a TLC experiment in the lab, a student determines the Rf value of caffeine to be 1.80. The student also notes that the solvent traveled 6.35 cm from the index mark. Based on your knowledge of the TLC technique, what can you conclude about this result?
The spot did not move from the index mark.
The Rf result reported by the student does not seem valid.
The caffeine spot traveled 4.0 cm from the index mark.
The solvent that the student used for this analysis was non-polar.
The Rf result reported by the student does not seem valid.
At 100°C, the equilibrium vapor pressure of water, methanol, and ethanol is 760 torr, 2625 torr, and 1694 torr, respectively. Which compound has the highest normal boiling point?
Water
Methanol
Ethanol
Water
Which of the following statements about fractional distillation are TRUE? [Select all that are TRUE].
Can ideally separate mixtures with comparable volatilities (small BP differences).
The larger the HETP (height equivalent to a theoretical plate) of the fractionating (packed) column, the more efficient it is.
It can determine the boiling points of components of a mixture of volatile liquids.
The distillation process involves several concurrent vaporization-condensation cycles.
Continual change in boiling temperature is observed as the distillation progresses.
Can ideally separate mixtures with comparable volatilities (small BP differences).
It can determine the boiling points of components of a mixture of volatile liquids.
The distillation process involves several concurrent vaporization-condensation cycles.
The main separation principle in the gas chromatography technique is through which of the following?
Absorption
Partition
Precipitation
Adsorption
Ion-exchange
Partition
In gas chromatography, the stationary phase is best described as;
The interior surface of an untreated capillary column that encounters a liquid mobile phase passing through the column
High boiling point, non-volatile liquid coated on the surface of finely divided solid packed within a column
Thin layer of silica or alumina particles are bound to a two-dimensional plate within a column
A chemically unreactive gas that comes in contact with a liquid mobile phase
High boiling point, non-volatile liquid coated on the surface of finely divided solid packed within a column
For each of the following purifications, identify situations where fractional distillation would be more suitable.
[Select all that apply]
Determining the boiling points of components in a mixture of volatile liquids
Preparing drinking water from the sea salty water
Effectively separating a 50:50 mixture of cyclohexane, boiling point 80°C from toluene, boiling point 111°C
Removing diethyl ether, boiling point 35°C, from p-dichlorobenzene melting point 175°C
Determining the boiling points of components in a mixture of volatile liquids
Effectively separating a 50:50 mixture of cyclohexane, boiling point 80°C from toluene, boiling point 111°C
The ratio of the amount of analyte in the stationary phase to the amount in the mobile phase.
Elution
Stationary phase
Chromatogram
Retention factor
Retention time
Retention factor
A series of peaks of different sizes produced by components in a mixture:
Elution
Stationary phase
Chromatogram
Retention factor
Retention time
Chromatogram
The time it takes after sample injection into the column for the analyte peak to appear as it exits the column.
Elution
Stationary phase
Chromatogram
Retention factor
Retention time
Retention time
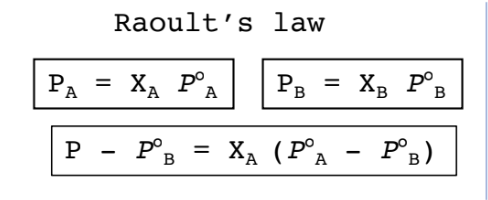
Raoult’s Law
Dalton’s Law
What torr number dictates lowest normal boiling point
The highest torr number out of all the options
When using a separatory funnel to separate immiscible liquids, which draining technique is applied for the bottom and top layers?
After draining out the bottom layer through the drain sten, the top layer is drained out through the drain stem.
After pouring out the top layer through the top (inlet), the bottom layer is drained out through the drain stem
After draining the bottom layer through the drain stem, the top layer is poured through the top (inlet)
After pouring out the top kayer through the top (inlet), the bottom layer is poured through the top (inlet)
After draining the bottom layer through the drain stem, the top layer is poured through the top (inlet)
The quantitative relationship between the vapor pressure of a component and its composition in a homogeneous liquid mixture is:
Real solutions
Raoult's law
Ideal solutions
Dalton's law
Distilland
Raoult’s Law
Solutions in which the interactions between like molecules are the same as those between unlike molecules are:
Real solutions
Raoult's law
Ideal solutions
Dalton's law
Distilland
Ideal Solutions
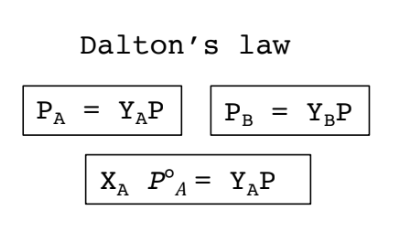
A law that describes the relationship between total pressure and the partial pressures of components of homogeneous liquid mixtures is:
Real solutions
Raoult's law
Ideal solutions
Dalton's law
Distilland
Dalton’s Law
For each of the following purifications, identify solutions where simple distillation would be more suitable?
Purifying, by distillation, drinking water from the sea’s salty water
Removing 3% diethyl ether (bp 35 degrees C) impurity from 25 mL ethyl acetate (bp 77 degrees C) solvent
Which statements about fractional distillation are false [SELECT ALL THAT APPLY]
A. Can ideally separate mixtures with comparable volatilities (small BP differences).
B. The larger the HETP of the dractionating (packed) column, the more efficient it is.
C. It can determine the boiling points of components of a mixture of volatile liquids.
D. The distillation process involves several concurrent caporization-condensation cycles.
E. Continual change in boiling temperature is observed as the distillation progresses.
The larger the HETP of the dractionating (packed) column, the more efficient it is.
Continual change in boiling temperature is observed as the distillation progresses.
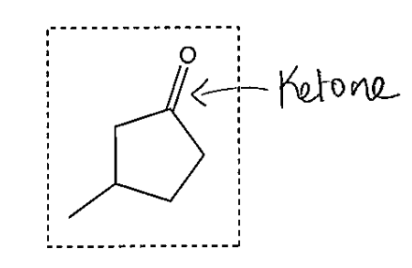
The compound below was spotted on a TLC plate and developed using a suitable solvent. Which visualization method would be appropriate for this compound?
A. Ultraviolet lamp
B. Chromic acid solution
C. Potassium permanganate solution
D. 2,4 dinitro phenyl hydrazine solution
Ultraviolet lamp
2,4 dinitro phenyl hydrazine solution
The process of a substance/compound traveling through the adsorbent carried by the mobile phase is
A. Elution
B. Stationary phase
C. Chromatogram
D. Retention factor
E. Retention time
Elution
The ratio of the distance traveled by the solute versus the distance traveled by the solvent in thin layer chromatography is called
A. Elution
B. Stationary phase
C. Chromatogram
D. Retention factor
E. Retention time
D. Retention factor
What purpose of the fractionating column in fractional distillation?
To condense the vaporized components into distillate
To analyze the mol % of each component
To allow for many vaporization-condensation cycles to occur
To measure the boiling point of the fractionated sample
To allow for many vaporization-condensation cycles to occur
What is the purpose of boiling stones in a distillation setup?
To measure the temperature of the vapors produced
To reliquefy the vapors and direct the distillate to the recieving flask
To prevent uneven boiling in the liquid distilland
To decolorize the liquid distilland
To ensure fractionation of components in the liquid mixture
To prevent uneven boiling in the liquid distilland
Which of the following factors would affect retention (Retardation) factor (RF) value in TLC chromatography? [SELECT ALL THAT APPLY]
The absorbent
Size of the TLC plate
Developing solvent system
Identity of the compound
All of the above
Developing solvent system
Identity of the compound
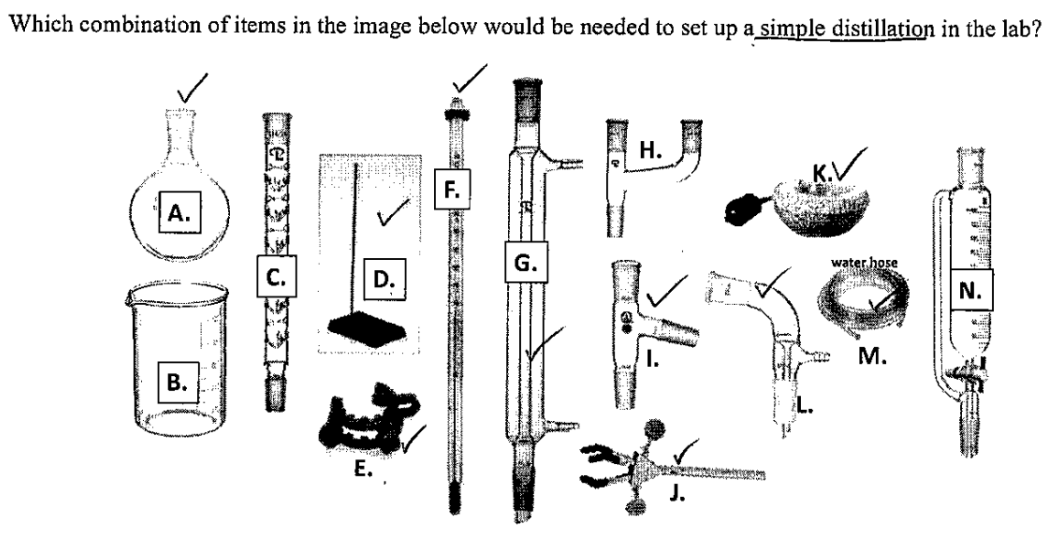
A + D + E + F + G + I + J + K + L + M

Still head/distillation head
What would happen to the retention time of a compound if we increased the length of the column.
Retention time increases
What would happen to the retention time of a compound if we increased the temperature of the column.
Retention time decrease
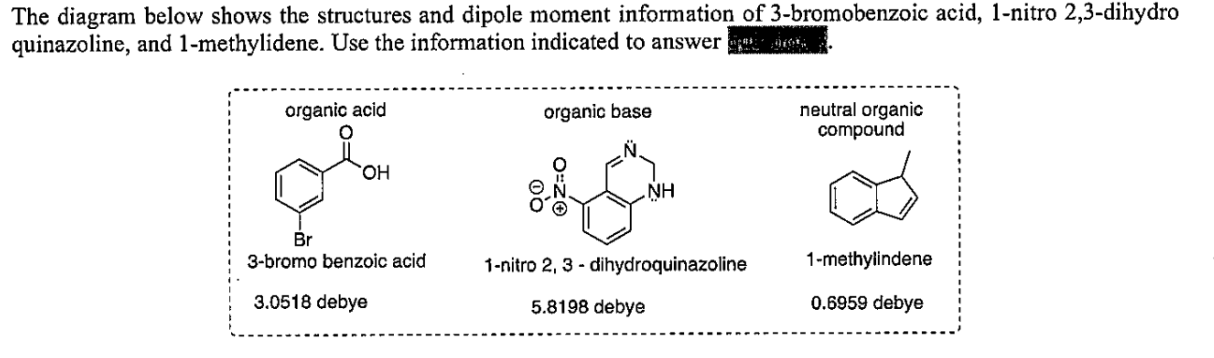
Suppose you start with a 1:1:1 by-weight mixture of the three compounds above. You dissolve 1 gram of this mixture in diethyl ether and spot a sample of the solution on a TLC plate and develop it. After the plate is developed, you see three spots. Which compound will have the smallest Rf value based on the information provided?
1-nitro 2,3-dihydro quinazoline
At 100°C, the equilibrium vapor pressure of water, methanol, and ethanol is 760 torr, 2625 torr, and 1694 torr, respectively. Which compound has the lowest normal boiling point?
Water
Methanol
Ethanol
Ethanol
Assume that you are provided with an 18 cm fractionating column having an HETP of 6 cm to distill a mixture of 84% toulene and 16% acetone. What would be the composition of toulene in the first drop of distillate?
30% toulene
54% toulene
70% toulene
16% toulene
90% toulene
30% toulene
Which compound will have the smallest Rf value based on the information provided?
The greater compount + debye = the smallest Rf