Biological Molecules
1/80
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
Molecules
Made of two or more atoms
Compound
If atoms in a molecule are different.
Element
If atoms in a molecule are the same.
Types of compounds (think bonding)
Ionic- metal and non-metal
molecular (covalent)- just non-metals
Ions
Charged particles through loss or gain of electrons from an atom.
Polar vs non-polar molecules
Polar- slight charge across molecule
Non-polar- no charge across molecule
Properties of ions and polar compounds
Attract oppositely charged particles.
Important structurally.
Properties of non-polar compounds
Lipid-soluble rather than dissolving in water.
Four most common elements making up human body mass.
Carbon, Hydrogen, Oxygen, Nitrogen
C, H, O: These are the main components of all organic molecules
C, H, O, N: Found in amino and nucleic acids
Calcium in human body + plants
ion
role in humans + plants
Ca2+
2%
Strengthens teeth, bones and cell walls in plants.
Phosphorus in the human body
PO4 3-
Present in cell membranes/ ATP/ Nucleic acids
Magnesium in human body
Mg2+
0.05%
Used for enzyme function (and chlorophyll function in plants)
Iron in human body
Fe2+
0.004%
Oxygen transport
Potassium in human body
K+
0.35%
Nervous impulse transmission
Sulfur in human body
SO4 2-
Some amino acids (for disulphide bonds in tertiary protein structure)
Organic compounds
components
origin
Always contain C, H. May contain O and/or N.
Usually produced by living organisms or from decay of dead organisms. E.g. crude oil
Inorganic compounds
Can also contain C, H, O, N.
Can be made without involvement of living organisms. E.g. CO2, H2O.
Biochemicals
Compounds made by and found in living organisms
3 types of biochemicals
Carbohydrates
Lipids
Proteins
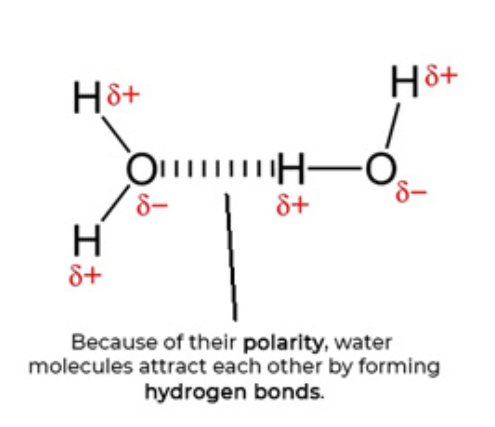
Structure of water molecule
Solid lines drawn showing bonds between H and O atoms.
Water is polar so hydrogen atoms have a partial positive charge and oxygen atoms hv a partial negative charge. These are shown by δ+, delta positive and δ− delta negative.
Due to their polarity, water molecules attract each other forming hydrogen bonds. This can be shown by a series of vertical lines.
Water as a universal solvent
Required in all biochemical reactions as they all take place in aqueous solutions, dissolved in water.
Because of waters polarity, water molecules are attracted to other water molecules and charged particles. (see properties of polar molecules). This helps charged particles dissolve in water.
Water as a metabolite in chemical reactions (condensation and hydrolysis reactions, photosynthesis and respiration)
Water as a reactant:
Large molecules are often broken down by the addition of a water molecule- hydrolysis
Photosynthesis
Water as a product:
Many small organic molecules can be combined with the loss of a water molecule- condensation reaction.
Aerobic respiration
Significance of ice being less dense than water.
This forms an insulating layer over the surface of aquatic habitats, so e.g. ponds don’t freeze solid and animals can still swim beneath in a comfortable temperature.
Significance of water as a liquid
Water is liquid at most temperatures; lots of heat energy is required to change state.
Can be used as a transport medium. E.g. in blood in mammals, in xylem in plants.
Significance of transparency of water
Light can pass through for aquatic plants to photosynthesise.
Significance of water’s high surface tension
Surface of water can support and become a habitat for organisms like pond skaters.
Significance of water’s high specific heat capacity
Lots of energy required to increase its temp by 1 degrees c.
The temperature of aquatic environments remains constant and thermally stable. Cells at optimum temp- don’t denature.
Significance of waters high specific latent heat of vapourisation.
Lots of energy needed to evaporate water.
Useful for organisms to cool down- frog panting and sweating, leaf transpiration.
Aquatic environments dont diminish easily.
Significance of water’s hydrogen bonds for strong cohesive and adhesive properties
Due to hydrogen bonds, water molecules stick together (cohesion) and stick to other charge substances. This means they can be placed under high tensile (pulling) forces and pulled through plants in transpiration.
Water support in plant cells
Water can act as a structural support by filling vacuoles in plant cells and upholding the plant
Water and buoyancy
Water has a high density as a liquid so can provide buoyancy to aquatic organisms allowing them to float.
Carbohydrates
All contain C,H & O
General formula: CnH2nOn E.g. C6H12O6
Monosaccharides
General types of monosaccharide
General formula
simple sugar carbohydrate monomers named by number of carbons.
E.g. triose, pentose and hexose.
Cn(H2O)n
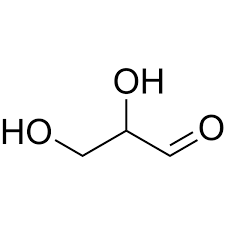
Glyceraldehyde
type of monosaccharide
structure
Triose
Structural isomers monosaccharides
Molecules with the same molecular formula but different structural formula. E.g. glucose, galactose, fructose.
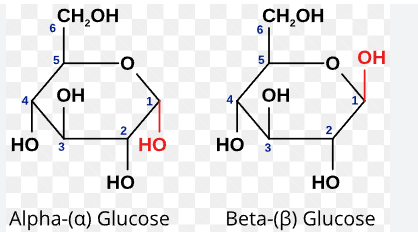
Alpha vs Beta glucose
Ring isomerism with differing position of OH group on carbon 1.
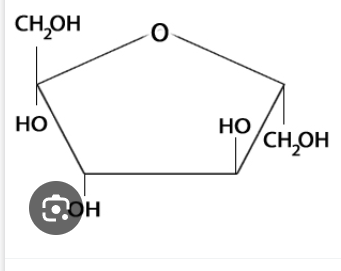
Fructose
Has central ring of 4 carbons with one oxygen atom, and CH2OH at carbons 1 and 6.
Structural isomer of glucose
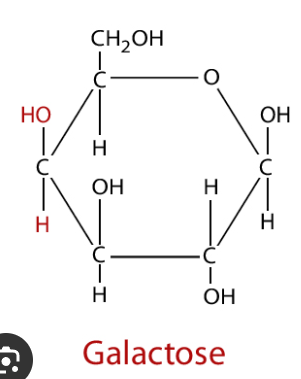
Galactose
Central ring of five carbons (like glucose) and one oxygen atom with a CH2OH group at carbon 6.
Disaccharides
components
type of reaction
bond formed
naming of bond
3 examples
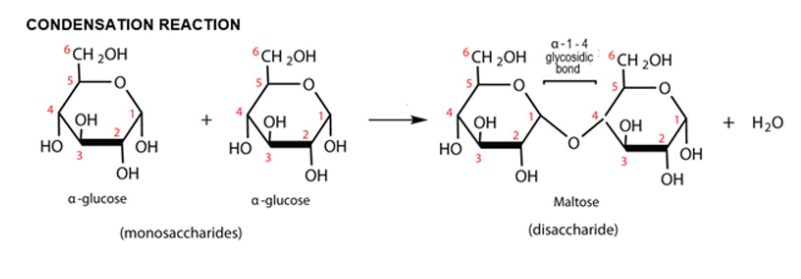
Sugars made of two monosaccharides joined by condensation reaction, forming a glycosidic bond. These bonds can be broken by hydrolysis reaction. The bond is named by the number of each carbon connecting the bond. E.g. Maltose, sucrose, lactose
Maltose
Disaccharide
two alpha glucose molecules with a 1,4 glycosidic bond.
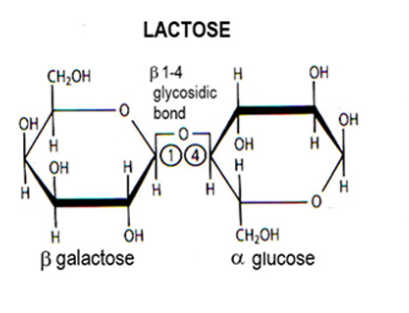
Lactose
Disaccharide:
Galactose + alpha glucose by 1,4 glycosidic bond.
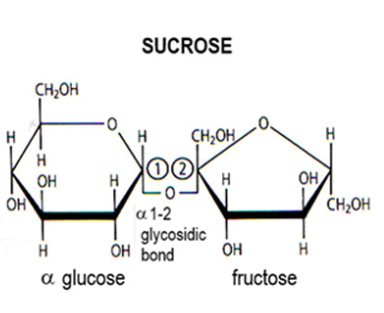
Sucrose
What type of molecule? Nature
Disaccharide:
Fructose + alpha glucose by 1,2 glycosidic bond.
Examples of polysaccharides- storage or structure?
Structure:
Cellulose (in plant cell wall)
Chitin (in fungi cell wall)
Storage:
Starch (in plants like potatoes)
Glycogen (in animals)
Storage polysaccharides similiarities
Can store and release glucose as necessary.
Made of chains of alpha glucose.
Starch structure
Made of two different polysaccharides: amylose and amylopectin
Amylose is a straight chain molecule made of alpha glucose with 1,4 glycosidic bonds. It forms coils into a helix through its hydrogen bonds between molecules.
Amylopectin is a branched molecule made of alpha glucose with 1,4 glycosidic bonds, and 1,6 glycosidic bonds at branching points.
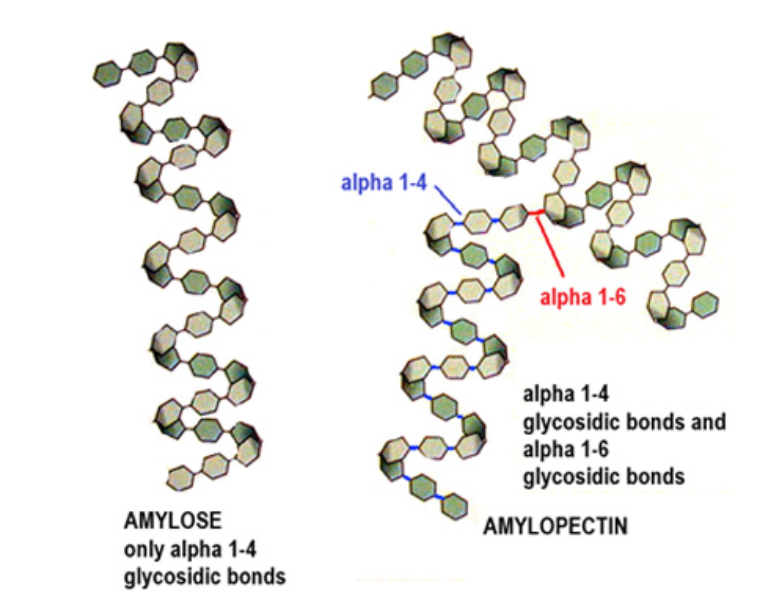
How is starch structure relative to function?
Due to coils from H bonds, starch is a compact molecule thats less soluble in water, so ideal to store glucose. It also means starch is osmotically stable.
Branches allow more ‘ends’ for hydrolysis to occur releasing glucose monomers, which can be used in respiration to produce ATP.
Glycogen structure
Branched chain of alpha glucose with both 1,4 and 1,6 glycosidic bonds. Similiar to amylopectin but more branched.
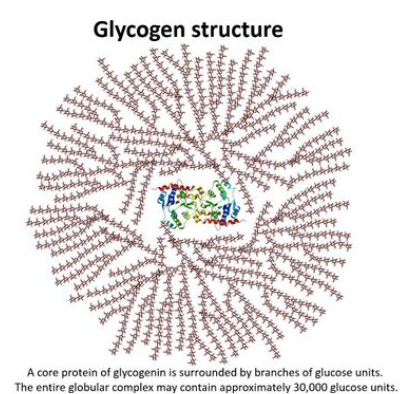
How is glycogen structure relative to its function?
Glycogen can form granules in cells and act as carbohydrate/energy store.
Branches allow more ‘ends’ for hydrolysis to occur releasing glucose monomers, which can be used in respiration to produce ATP.
Cellulose structure
Made of beta glucose molecule with 1,4 glycosidic linkage between adjacent molecules. This means each adjacent molecules are rotated 180 degrees so OH groups are aligned and a water molecule can be removed.
The rotating of alternate molecules causes H bonds to not form between glucose molecules in the same chain but between glucose molecules in separate chains. This is formation of cross linkages which hold chains together forming long threads called microfibrils.
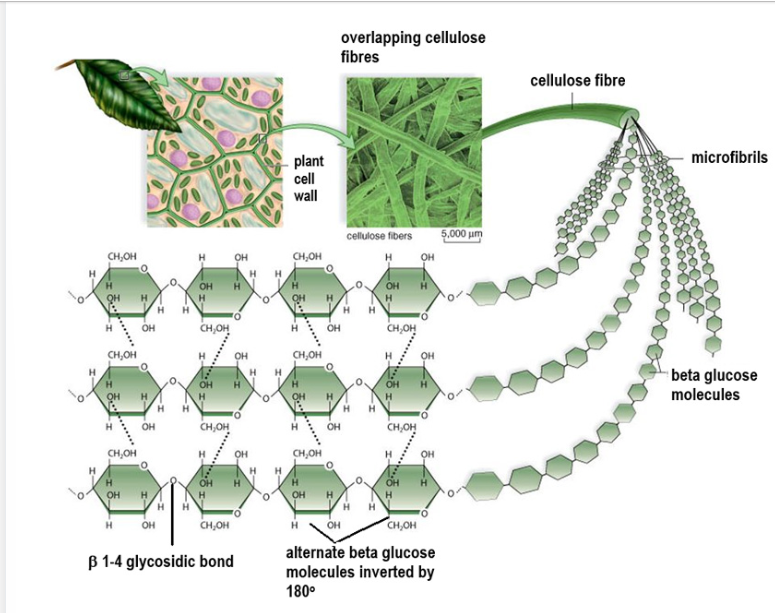
How does cellulose structure relate to its function?
Makes cellulose completely insoluble so increases stability.
Microfibrils are layed in overlapping layers in plant cell walls, making it difficult to digest and giving cellulose high tensile strength so is difficult to break when stretched (less likely to burst due to excess water intake).
Chitin structure
Not a true polysaccharide, as it has side groups containing N- instead its a heteropolysaccharide.
Has similar structure to cellulose; made of beta glucose with alternating rotated glucose molecules and hydrogen bonds between glucose molecules of different chains.
Due to presence of N, more hydrogen bonds can form.
How does chitin structure relate to its function?
More hydrogen bonds means greater tensile strength.
What are Reducing sugars? 3 examples
Carbohydrate that act as reducing agents by donating electrons or hydrogen atoms. E.g. glucose, lactose and maltose
Food test experiment: Reducing sugars
1) Put 2cm cubed glucose solution into boiling tube
2) Add an equal volume of Bendicts reagent
3) Heat in a water bath for 5 mins
Positive result turns mixture from blue to brick red.
Non-reducing sugars
Do not have the ability to reduce other substances by donating electrons or hydrogen atoms. E.g. sucrose.
Food test experiment: Non-reducing sugars
1) Put 2cm cubed sucrose into boiling tube
2) Add 2 drops dilute HCl
3) Place in water bath for 2 mins
4) Add 2 drops of dilute NaOH
5) And 2cm cubed Benedicts reagent
6) Place in water bath for 5 mins
Positive result- blue to brick red.
Lipids
Organic molecule with C,H & O. They have a high proportion of CH2 groups.
They also have a low solubility in water but high solubility in organic solvents like ethanol.
Triglycerides
what molecules does it form? what determines this?
what is it made up of? what bonds are formed?
Molecules that form fats or oils.
Lipids with long hydrocarbon chains are likely to be fats (solid at room temp). Lipids with short hydrocarbon chains are likely to be oils (liquid at room temp).
They are made from glycerol, combined with three fatty acids through a condensation reaction with the release of 3 water molecules, forming ester bonds.
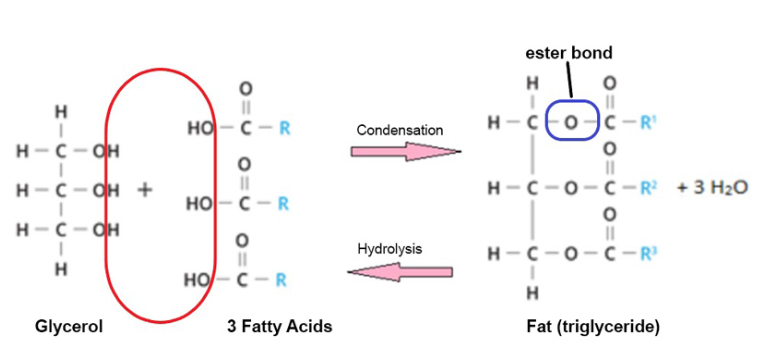
Functions of trigylcerides (4)
1) Efficient energy storage molecules. Store 38kJ of energy.
2) Good thermal insulators- protection for organs.
3) Fats provide buoyancy for aquatic animals as they are less dense than water.
4) Some animals spread oil for waterproofing because fats are hydrophobic and repel water.
Saturated fatty acids
What substance if often formed?
Only single carbon to carbon bonds with maximum hydrogen atoms.
Generally form fats as straight chains can pack close together forming forces of attraction between molecules so more energy is required to break the bonds so higher melting point.
Unsaturated fatty acids
Contains one or more carbon to carbon double bonds between carbon atoms, so fewer hydrogen atoms.
Usually oils at room temperature as double bonds form kinks so chains cannot pack as close together. Therefore weaker forces of attraction between molecules so lower melting point.
Phospholipids
Essential component in cell membranes.
Made of glycerol, a hydrophillic charged phosphate head and two hydrophobic fatty acid tails.
Functions of phospholipids
Form phospholipid bilayer in cell membranes.
Affect the fluidity of the cell membranes. Saturated fatty acids make membrane least fluid, unsaturated fatty acids make membrane most fluid.
LDL
Low-density lipoprotein cholesterol levels.
Bad cholesterol leading to blockages and eventually heart disease.
HDL
High density lipoprotein (good cholesterol)
Effect of saturated fat on body health
Where are they found?
Increases total cholesterol (waxy substance found in blood) and LDL. Best to consume in moderation.
Found in: red meat, whole milk, cheese, coconut butter, processed meat, many baked goods and deep fried foods.
Effect of polyunsaturated fats on body health
Where are they found?
Essential for the body as it is not produced by our bodies. It lowers LDL.
Found in: most cooking oils, pumpkin seeds, pine nuts, sesame seeds, fatty fish.
AKA: omega-3, omega-6
Monounsaturated fat
Considered as healthy fat: Lowers LDL and maintains HDL.
Found in: olive oil, avacado, avacado oil, most nuts and nut butters
Practical food test for lipids
1) Mix fat/oil with 5cm cubed of ethanol in a boiling tube.
2) Shake the tube and pour the mixture into another boiling tube with equal volume of water.
Positive result goes from clear to milky.
What is the bond between fatty acids and glycerol called?
Ester bonds
Amino acid
Building blocks for proteins.
There are 20 different amino acids.
It has 4 functional groups: Amine group (NH2), Carboxylic acids -(COOH), a hydrogen atom (H) and a variable group (R).
What is the bond formed between amino acids called
Peptide bonds formed from condensation reactions.
Primary protein structure
Specific sequence of amino acids in a polypeptide chain.
Secondary protein structure
Hydrogen bonds form between amine and carboxylic polar groups on amino acids. These make the polypeptide chain and fold into a new shape which is commonly an alpha helix or a beta pleated sheet.
Structural role of alpha helices
Used for fibrous proteins where several alpha helices coil together to form an insoluble rope like arrangement. E.g. collagen in skin
Tertiary structure of proteins
Forms the basic 3D shape of the protein.
Dependent on the properties of the R groups, 3 types of bonds can form, twisting the polypeptide chain further to form its 3D protein shape:
Hydrogen bonds forming between polar variable groups
Ionic bonds between charged variable groups. Can interact with water helping protein dissolve.
Disulphide bridges formed from two variable groups containing sulfur atoms. These covalent bonds are strong and more difficult to break.
Globular proteins
Formed from additional folding to a protein in its tertiary structure, with charged groups on the outside and hydrophobic parts on the inside of the protein.
Examples include:
Enzymes- active sites to bind to substrate.
Antibodies- sites binding to antigens.
Hormones- sites binding to specific receptors.
Quaternary protein structure
Proteins made of 2 or more polypetide chains combined, each chain having its own primary, secondary and tertiary structure.
Globular proteins with quaternary structure and metabolic functions.
Formed by disulphide bridges between polypeptides
insulin
haemoglobin
What are two fibrous proteins with quaternary structure and structural functions?
Formed by hydrogen bonds between polypeptides.
collagen
keratin
Protein food test practical
1) Add 2cm cubed albumen for protein solution to a test tube
2) Add biurets reagent to the boiling tube.
3) Invert the tube once, covering the mouth of the tube.
Positive result shows colour change from blue to purple.