1-Naming Alkanes
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
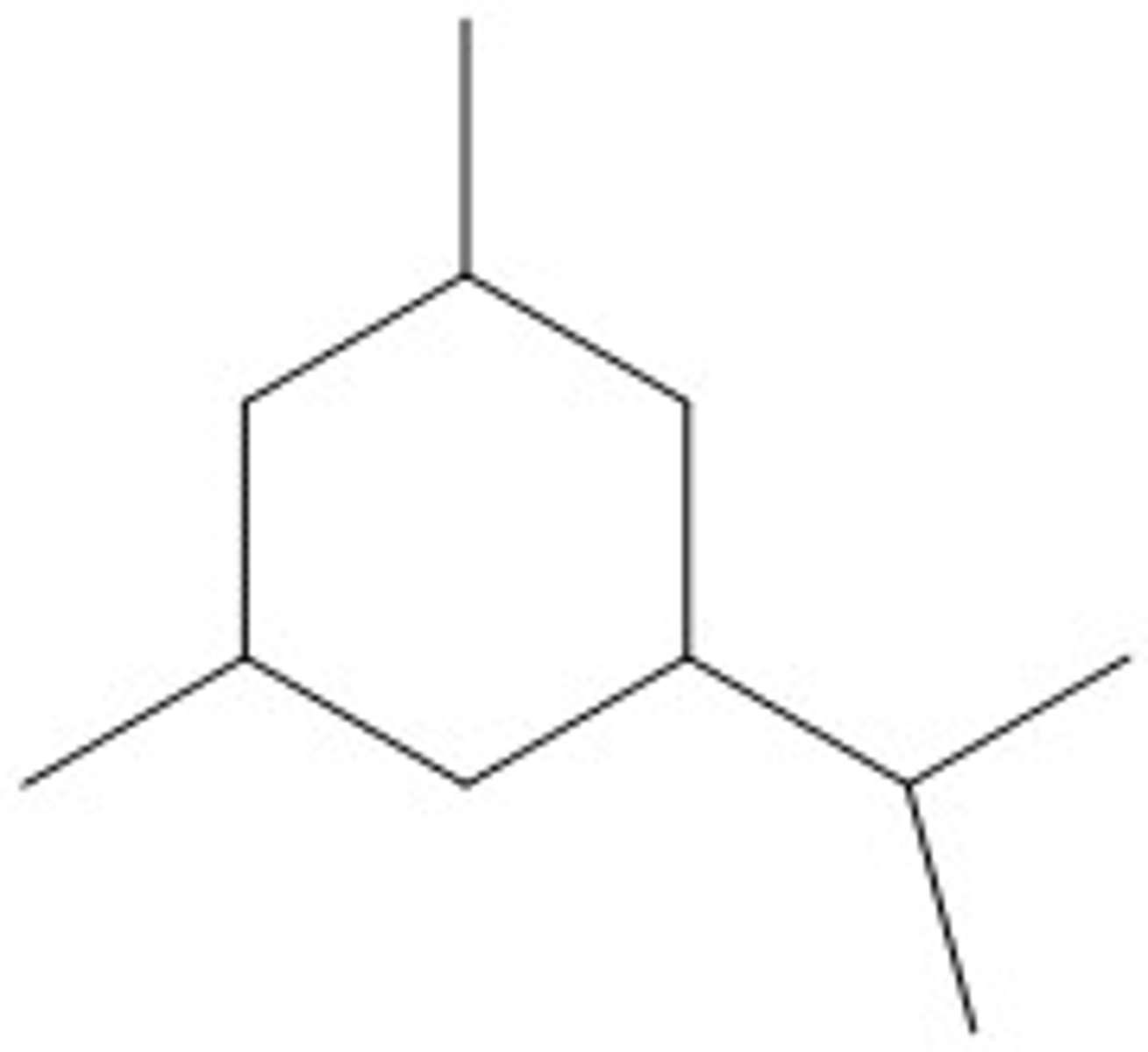
1-Isopropyl-3,5-dimethylcyclohexane
.
Butane

Cyclopropane
2,2-Dimethylpentane
Pentane
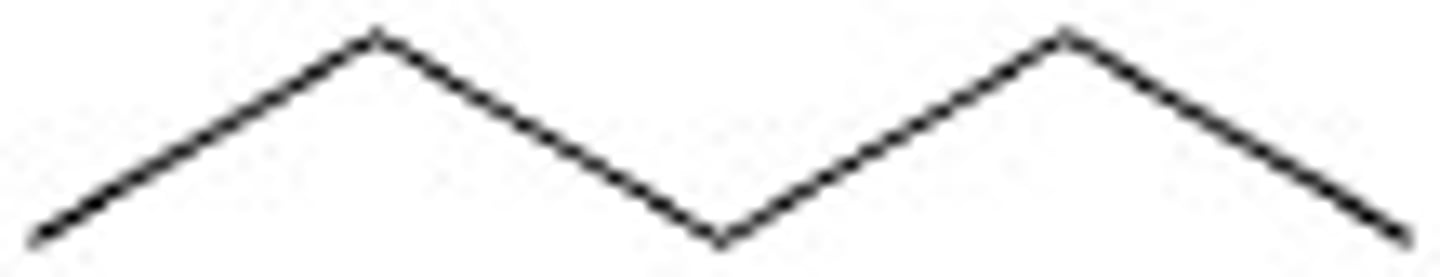
Propane
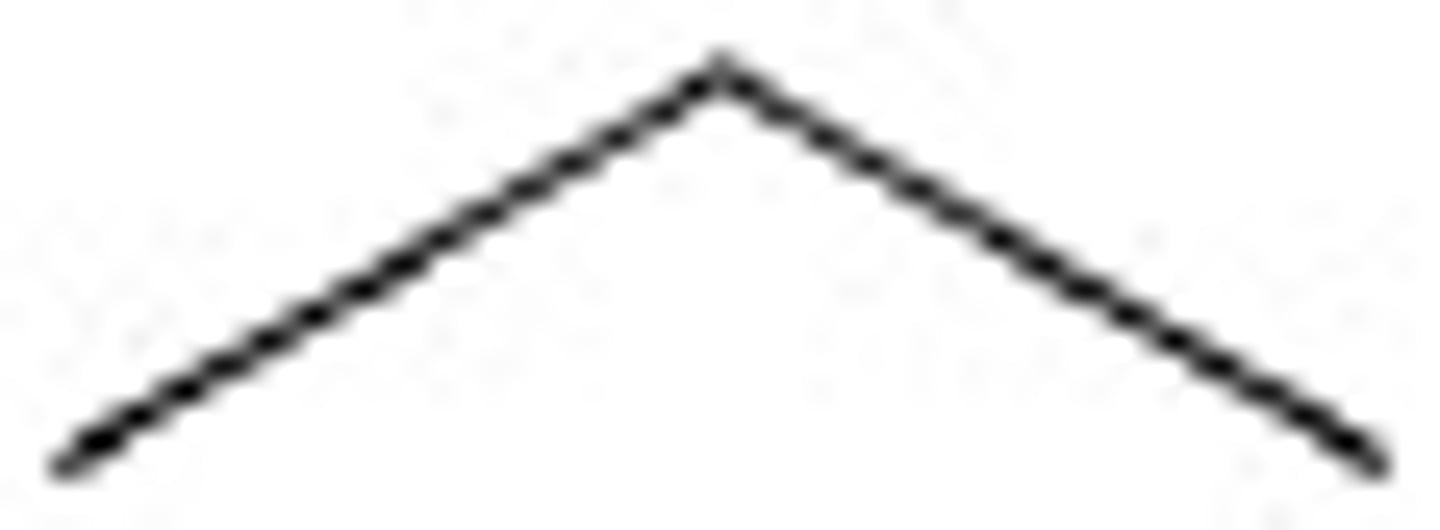
2-Methyloctane
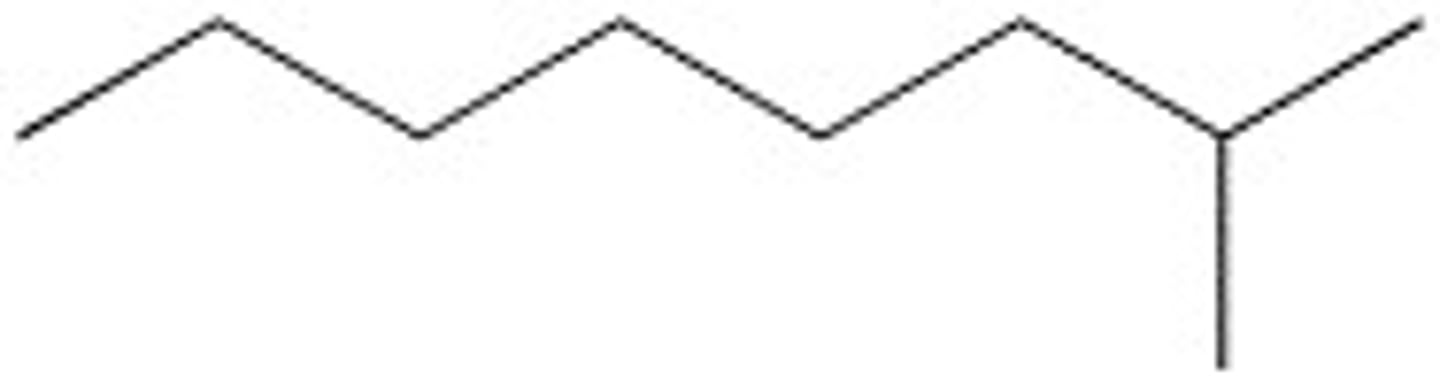
2,2,3-Trimethyloctane
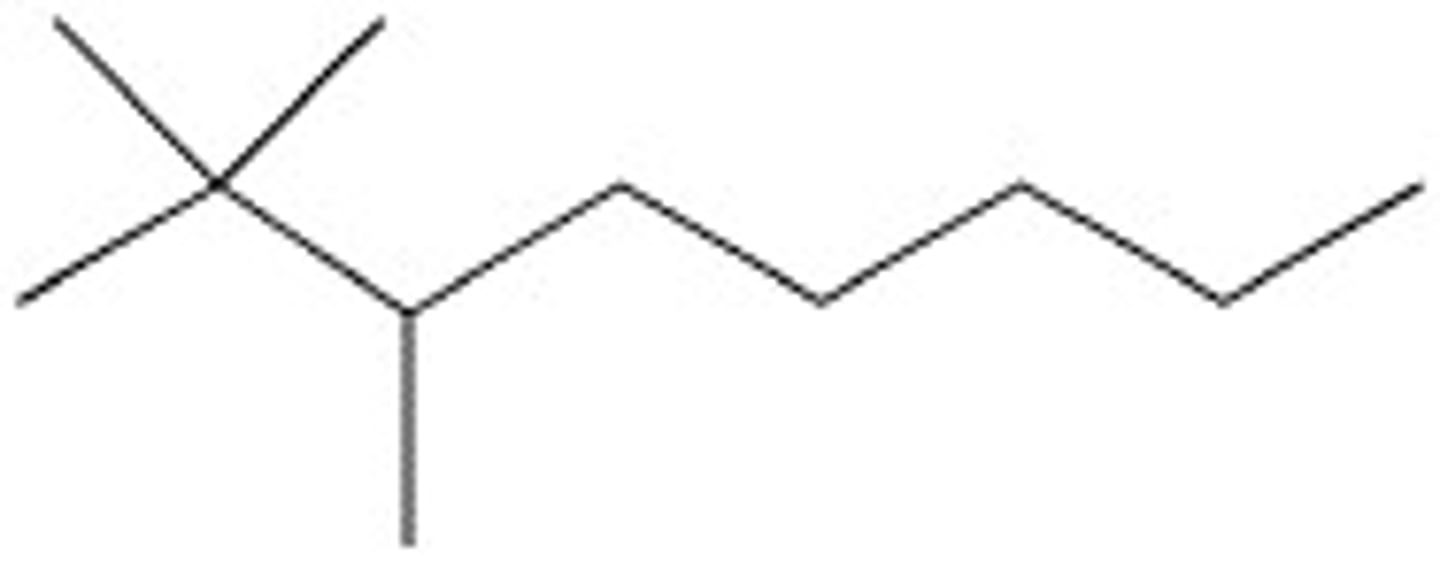
Cyclopentane
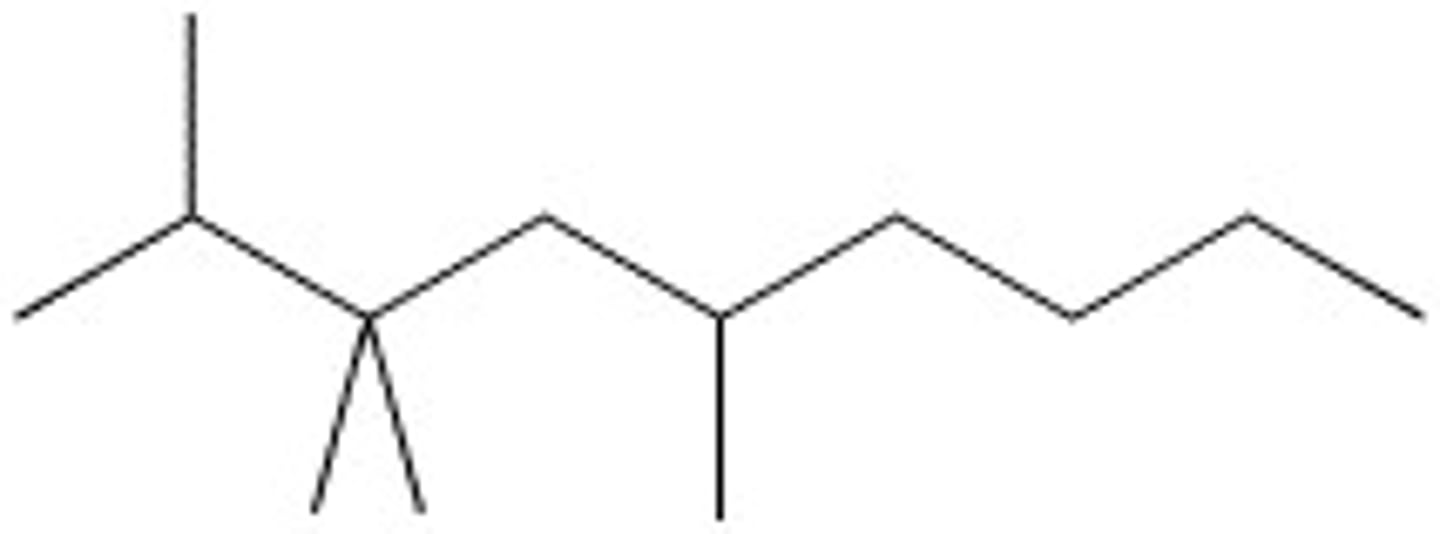
3,3-Diethyl-2,5-dimethylnonane
.
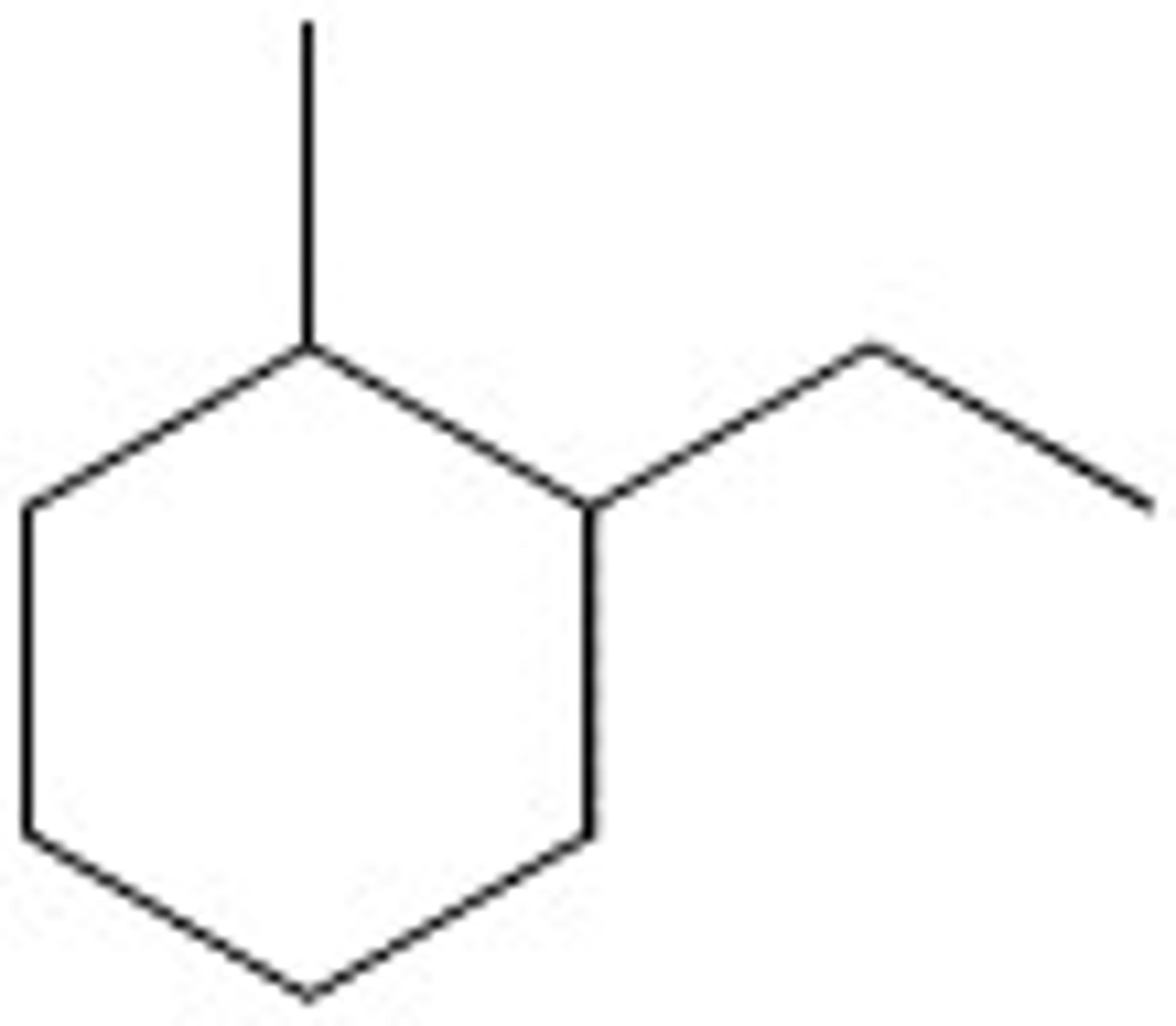
1-Ethyl-2-methylcyclohexane
.
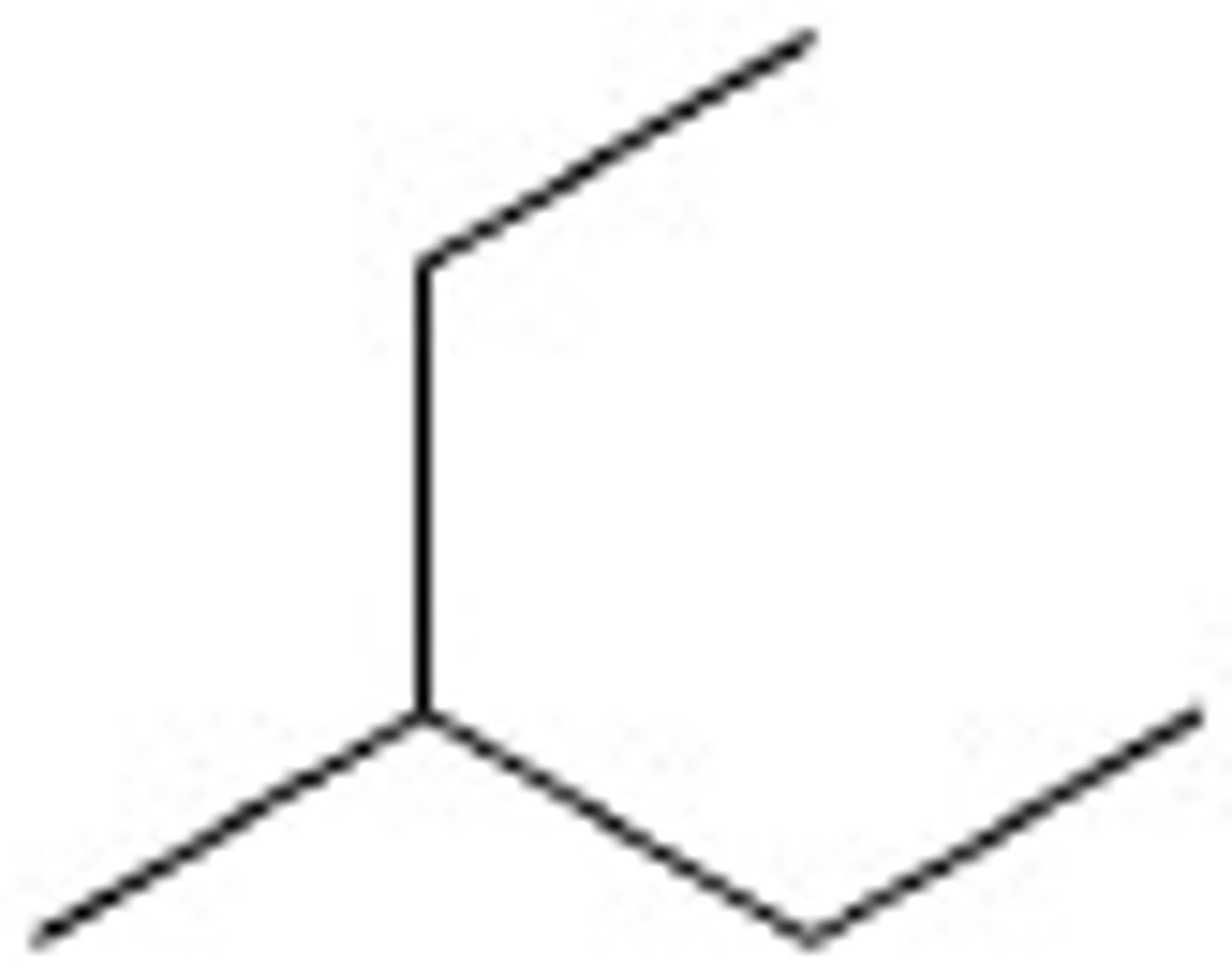
3-Methylpentane
Hexane

2-Methylbutane
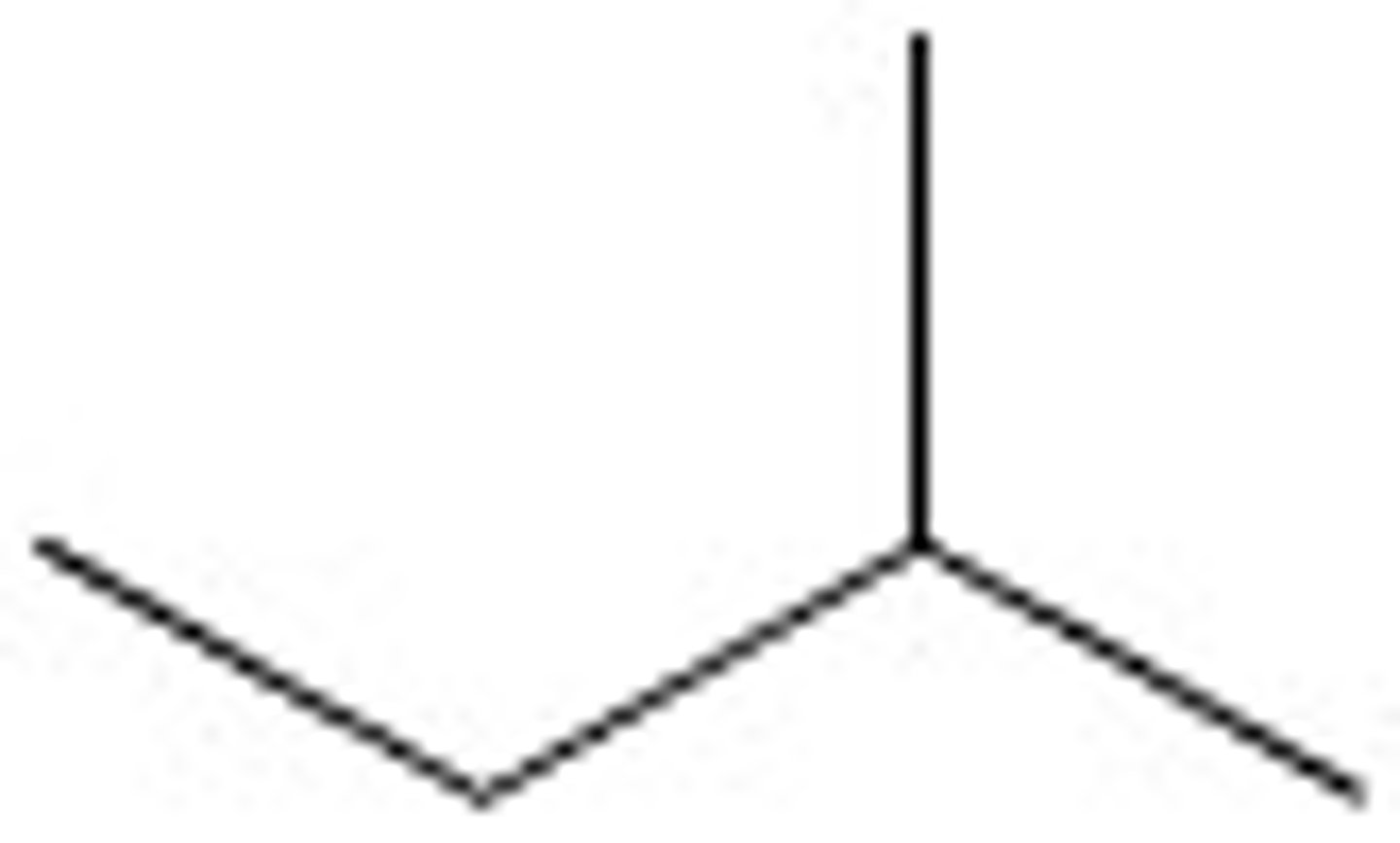
2,2-Dimethylbutane
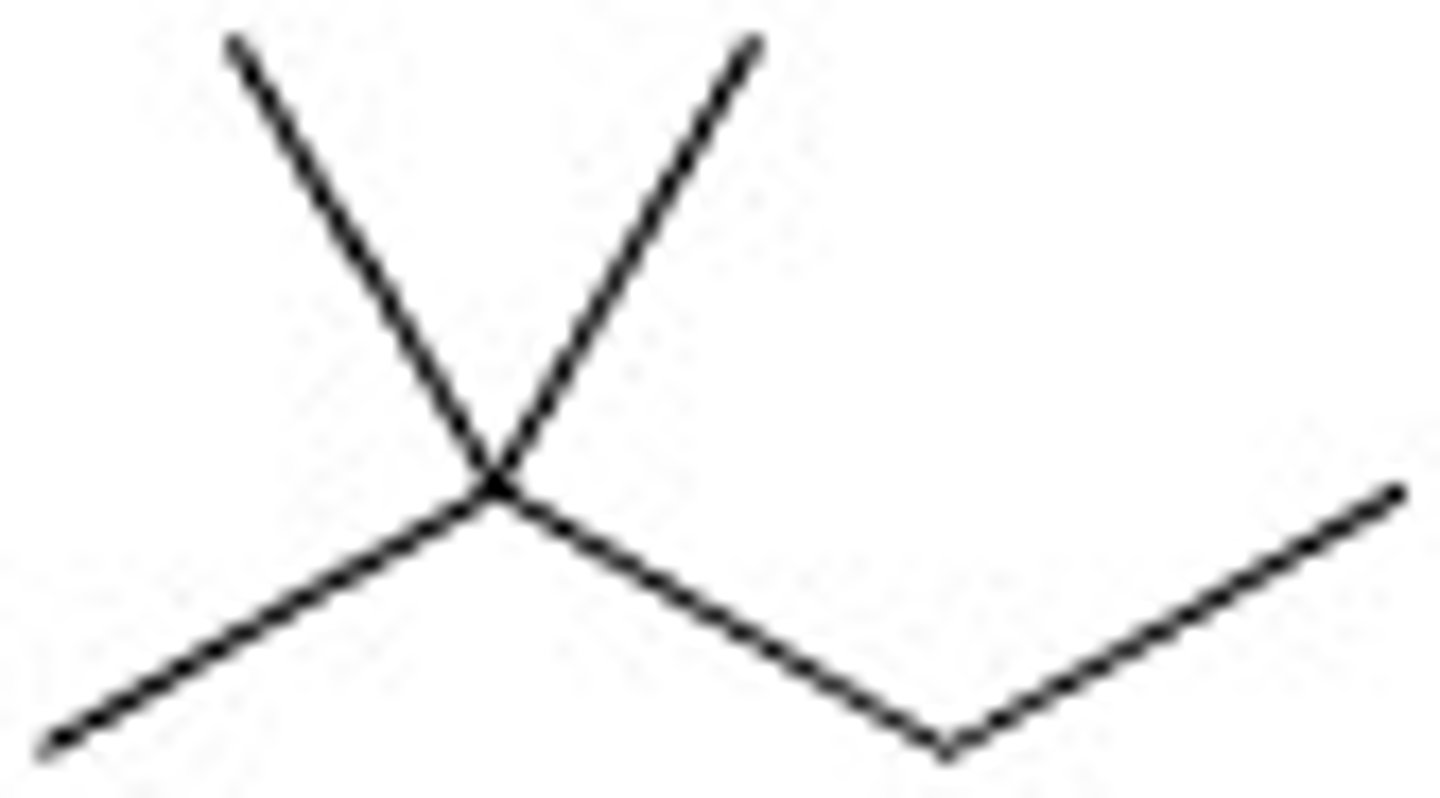
3,4-Dimethylhexane
.
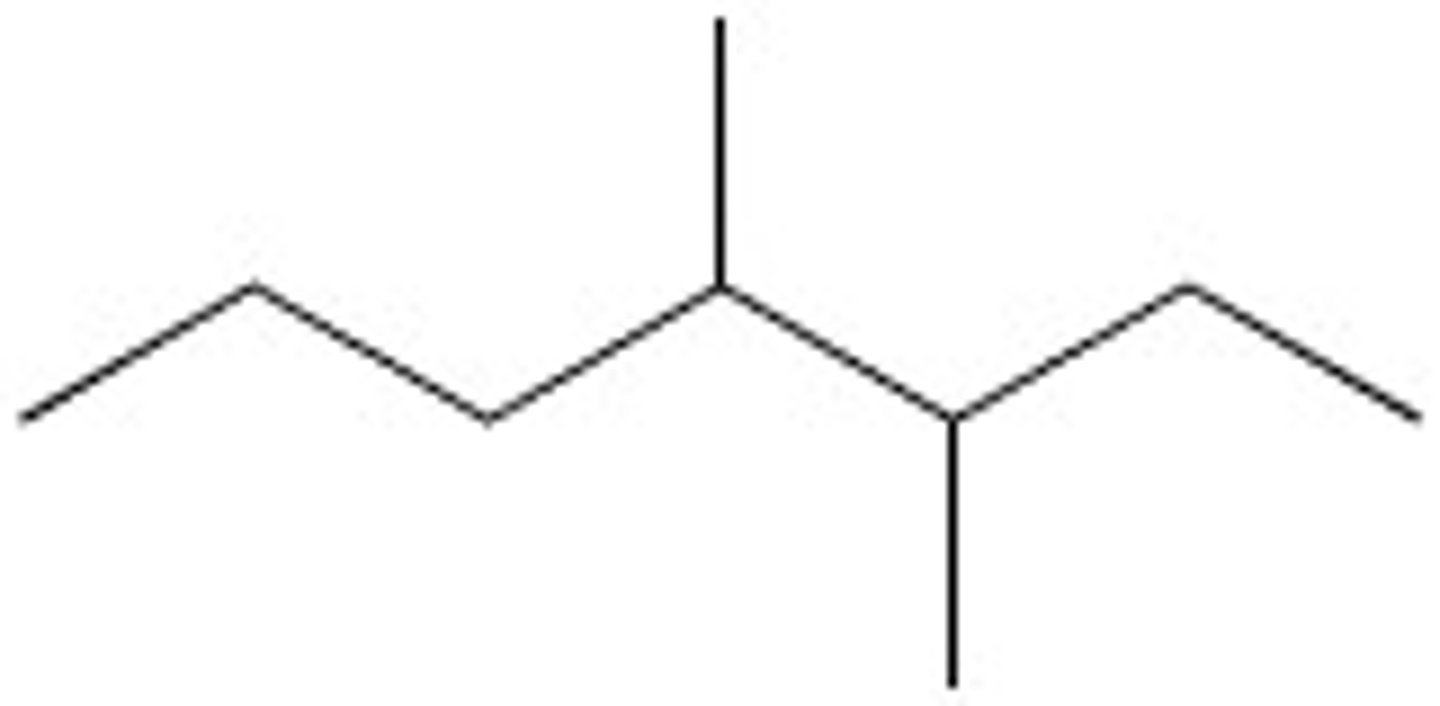
Octane

Heptane
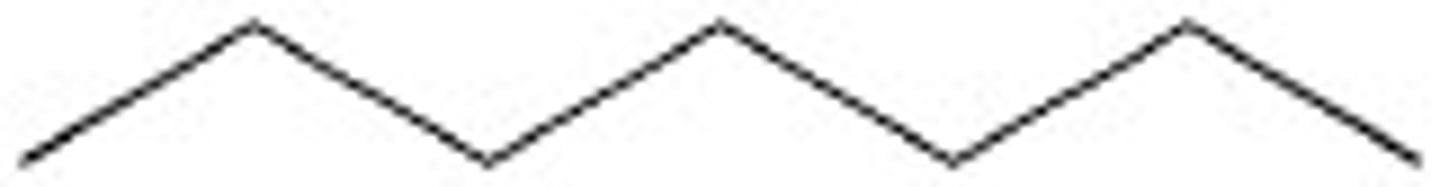
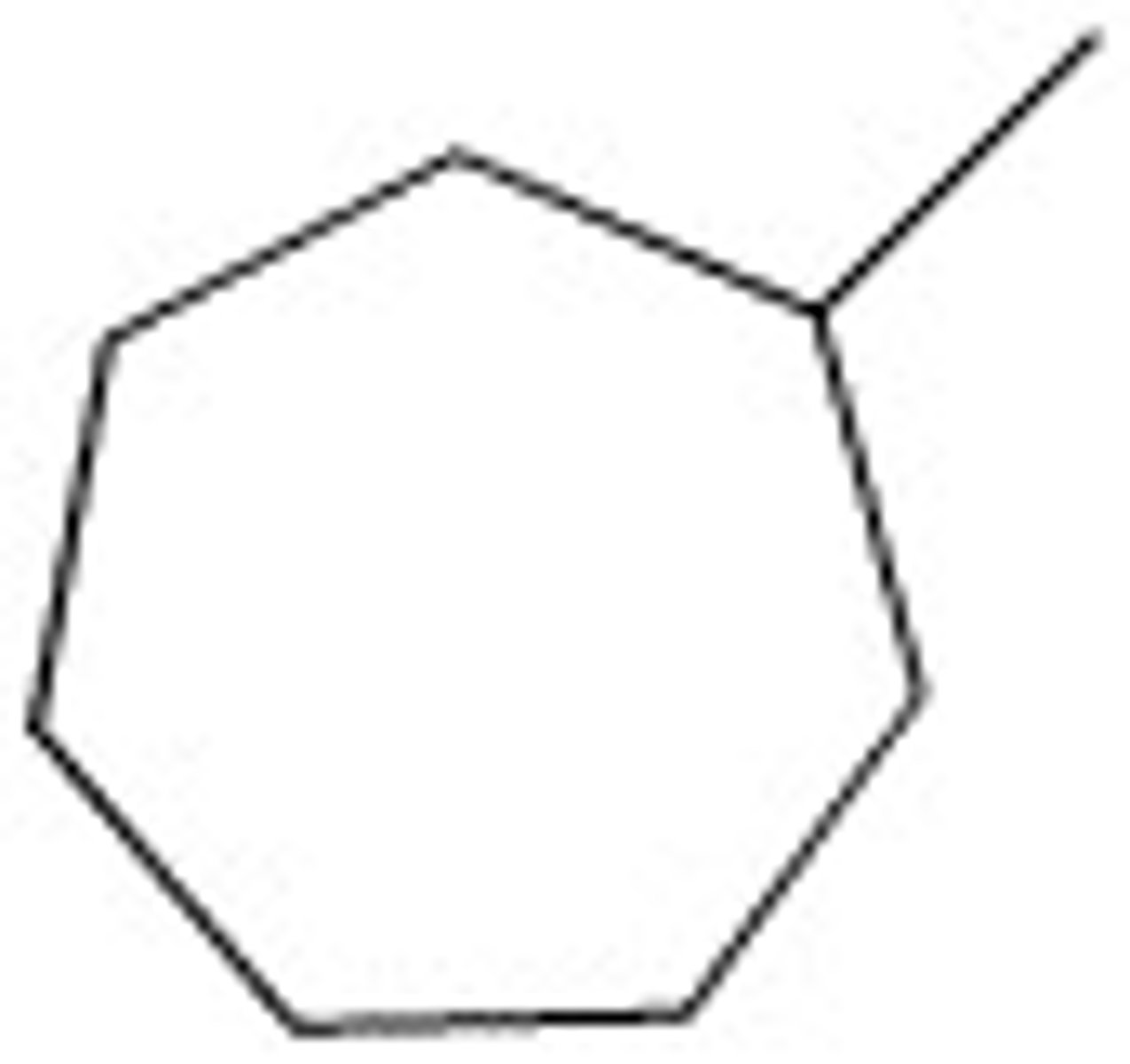
Methylcycloheptane
.
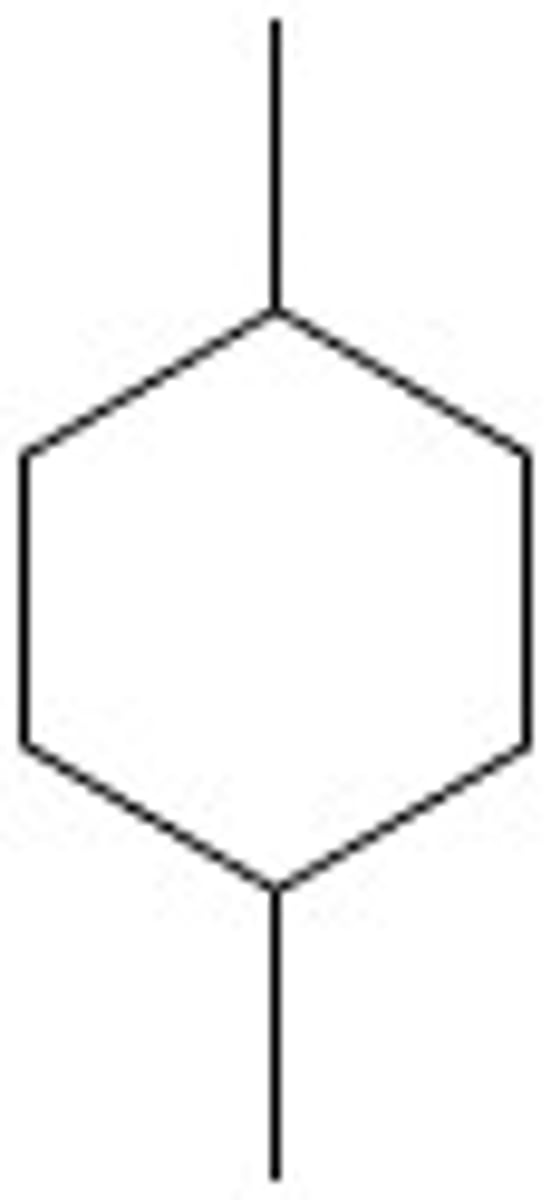
1,4-Dimethylcyclohexane
.
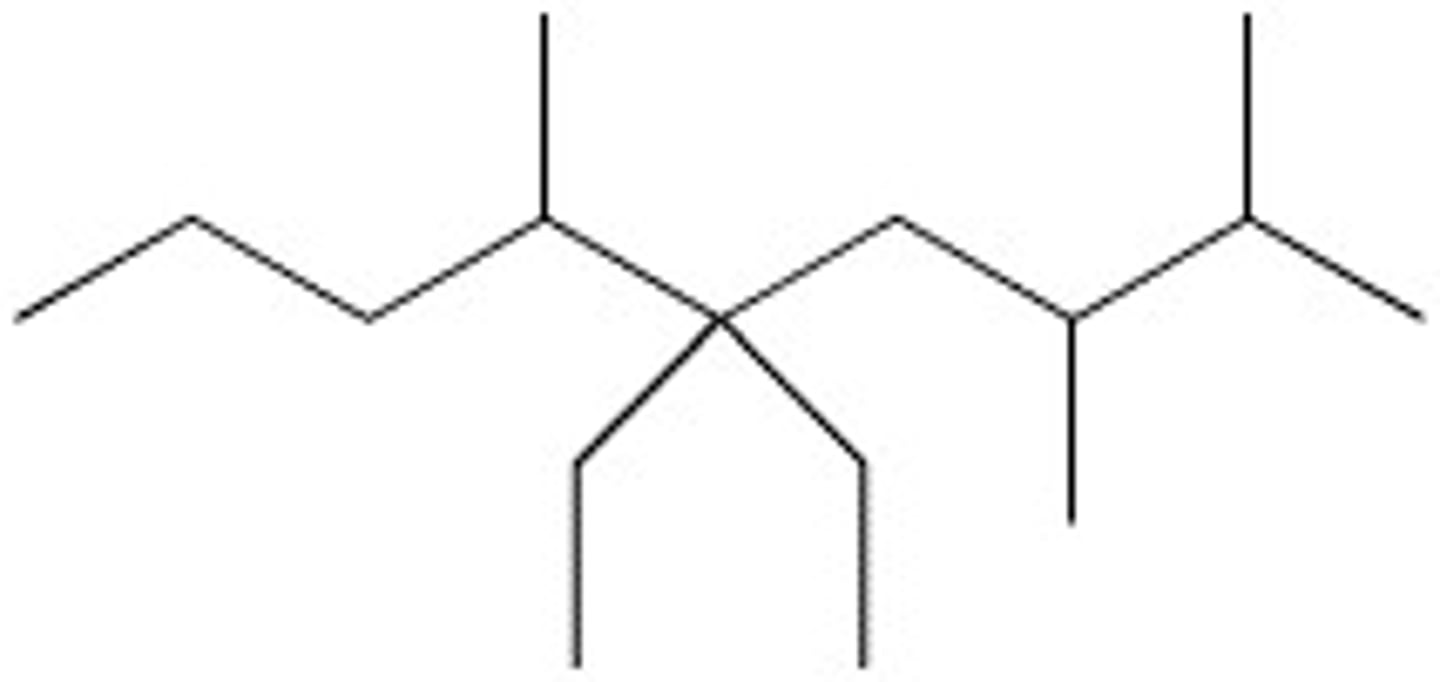
5,5-Diethyl-2,3,6-trimethylnonane
.
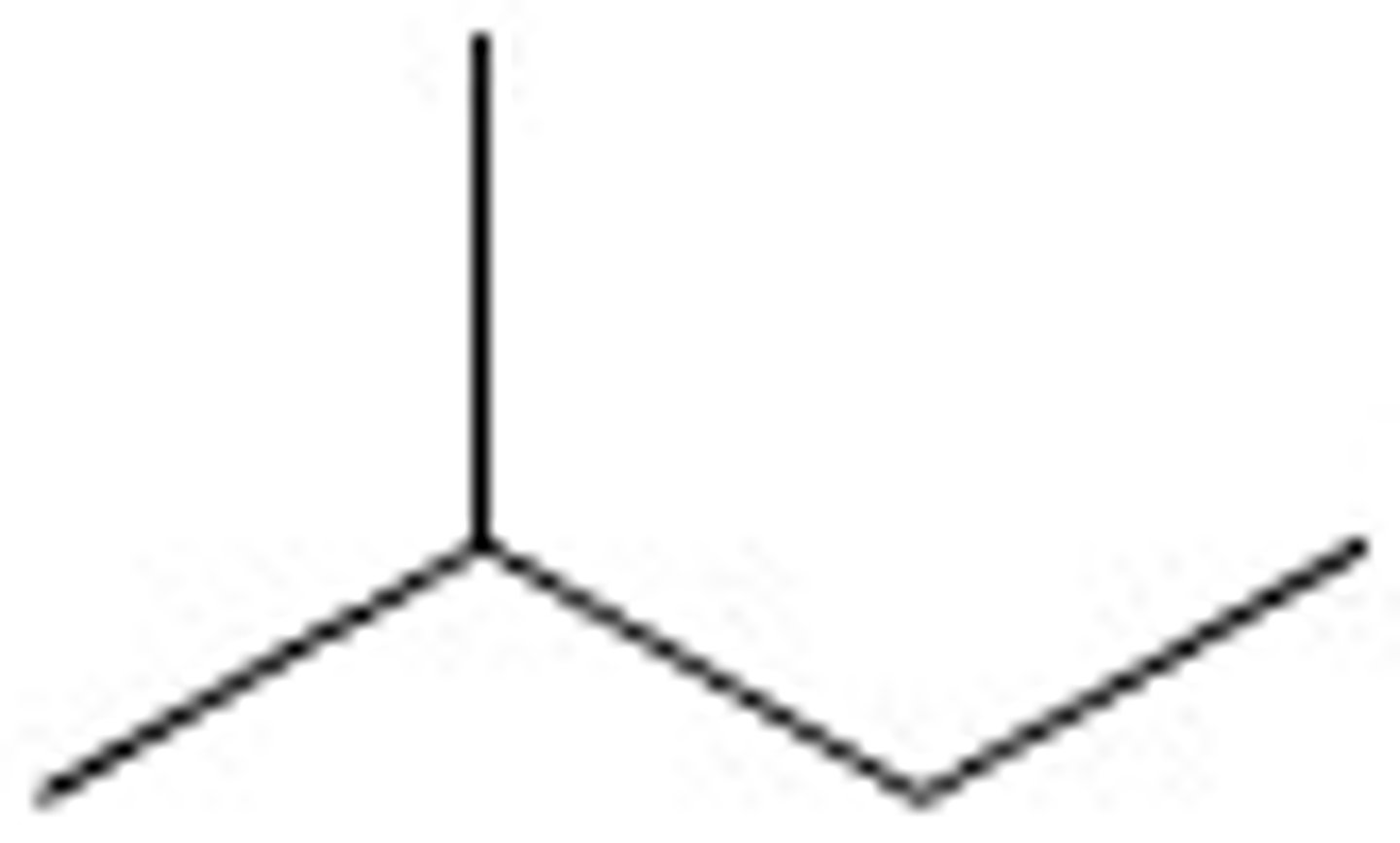
2-Methylbutane
C6,H14
How Many Carbon?
How Many Hydrogen?
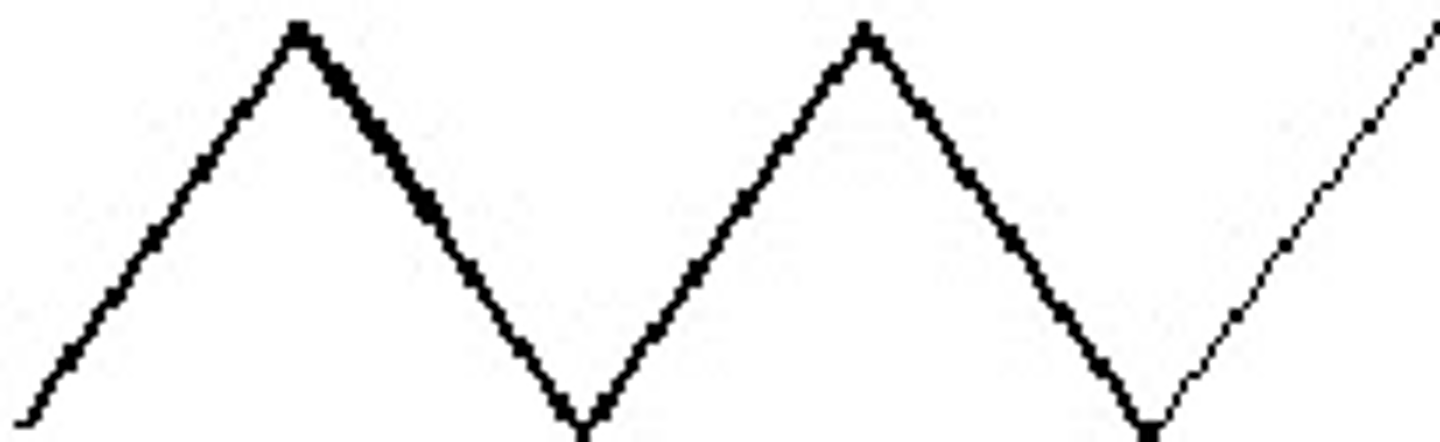
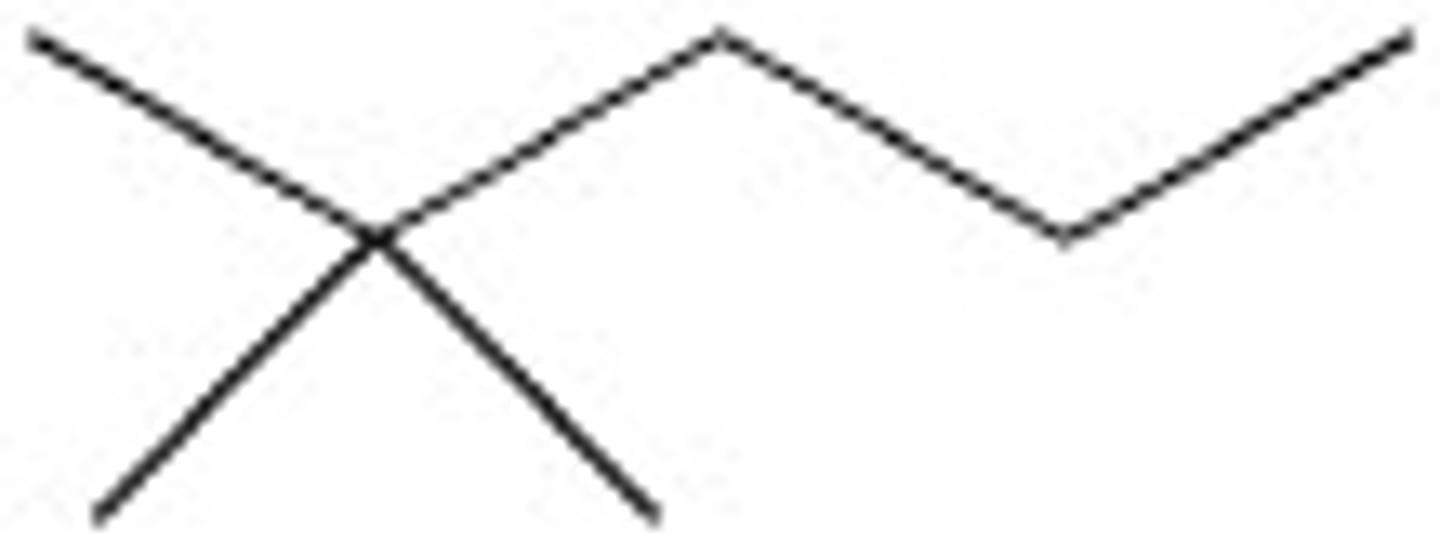
C6H12
Determine the chemical formula for this molecule
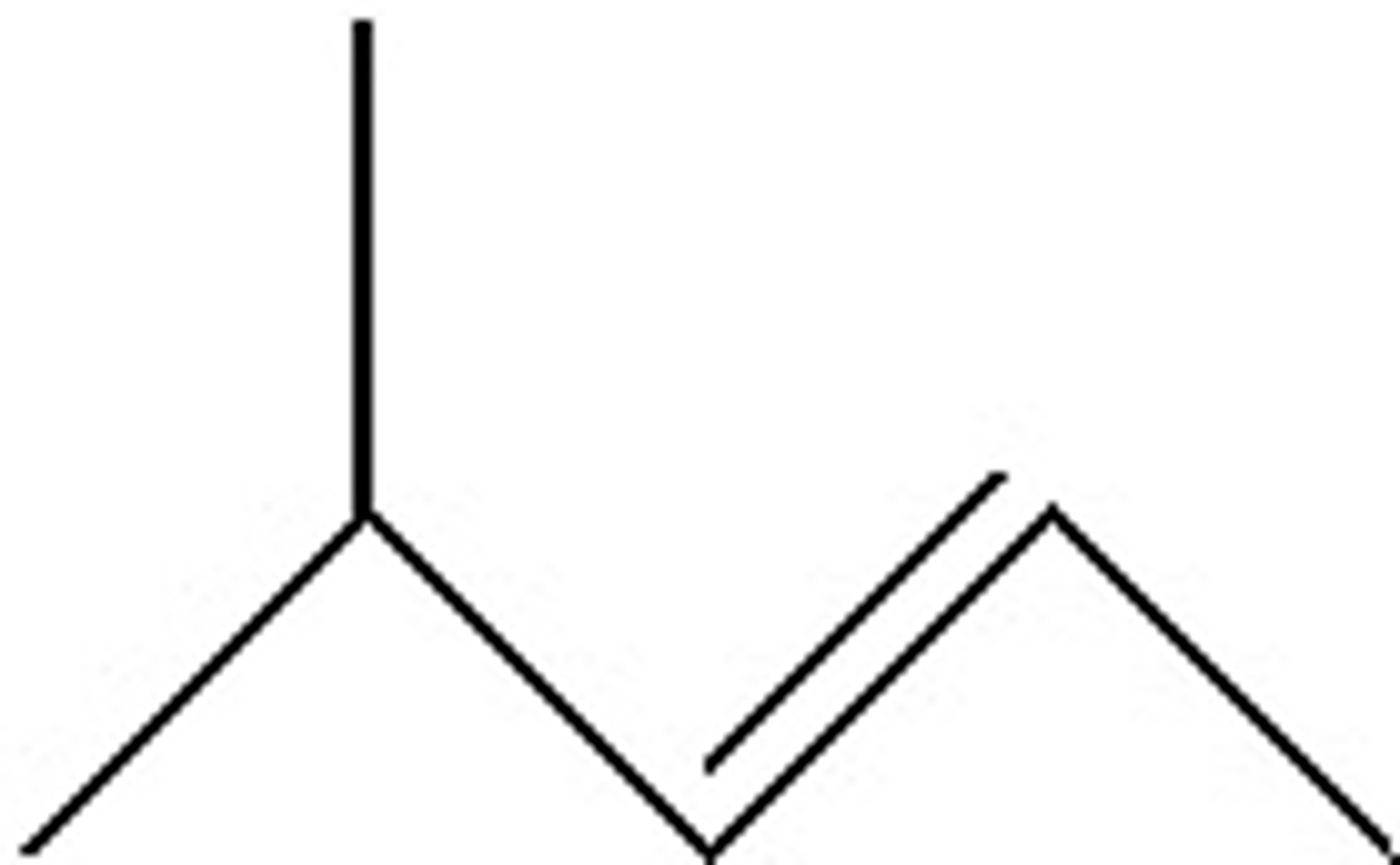
A,C
Several statements are given below. Choose all of the statements that would be TRUE for the molecule propane.
A) In complete combustion it yields three molecules of carbon dioxide
B) Its chemical formula is C3H6
C) It is a gas at room temperature and standard pressure
D) It is the simplest hydrocarbon
A) Structural Isomers
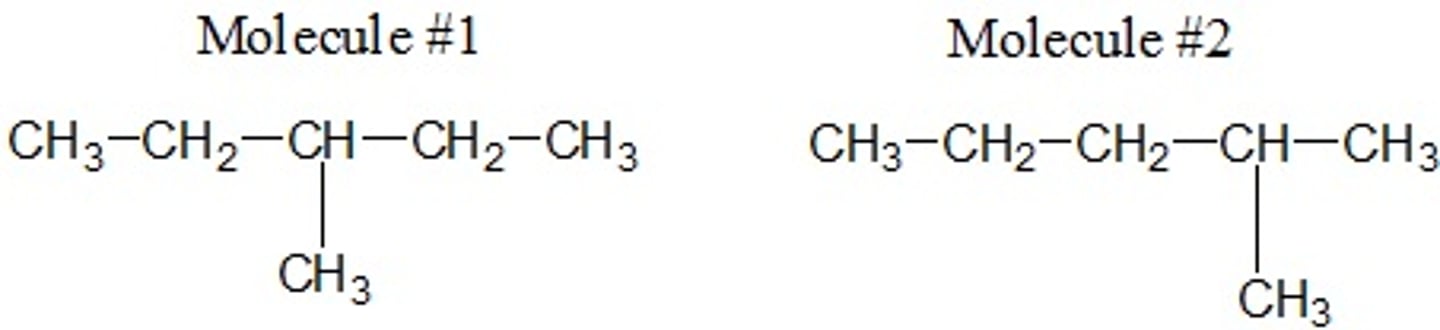
Which of the following best describes how these two molecules are related to each other? Choose the best answer.
A) Structural (constitutional) Isomers
B) Geometric Isomers
C) None of these options
D) Conformers
A) Structural Isomers
Which of the following best describes how these molecules are related to each other? Choose the best answer.
A) Structural (constitutional) Isomers
B) Conformers
C) Geometric Isomers
D) None of these options
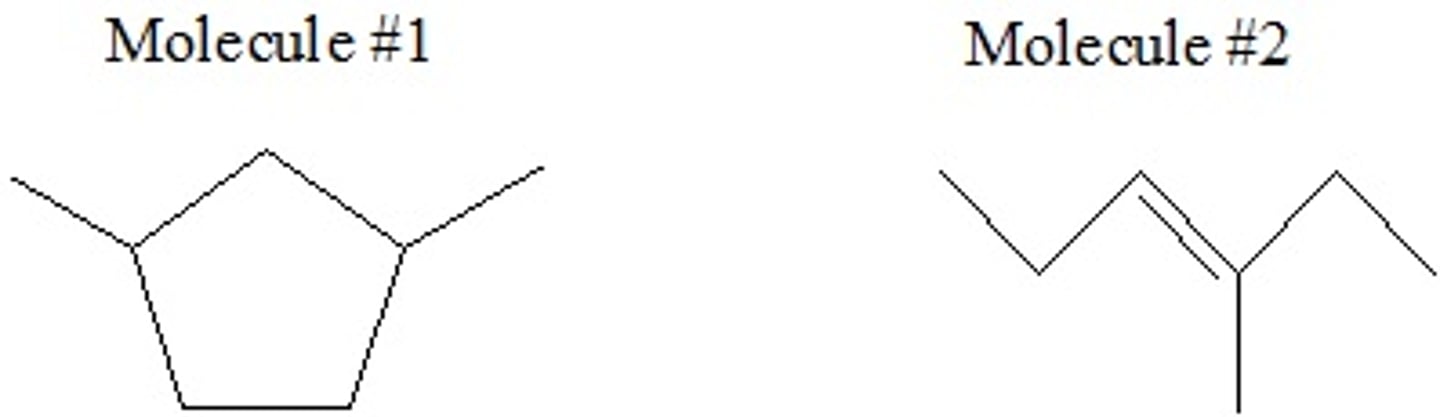
3-ethyl-5-methylheptane
What is the Iupac name?

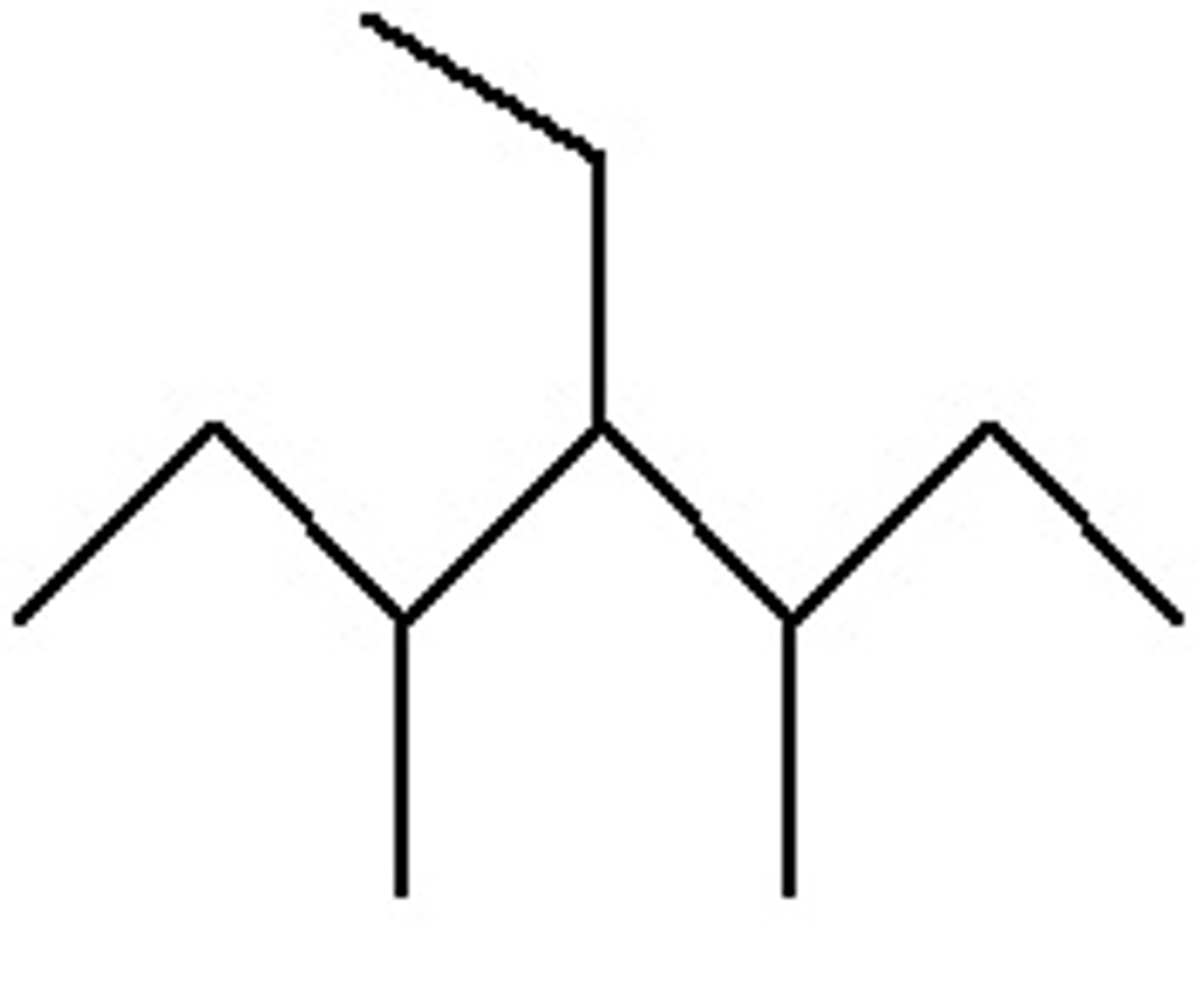
4-Ethyl-3,5-dimethylheptane
What is the Iupac name?
A
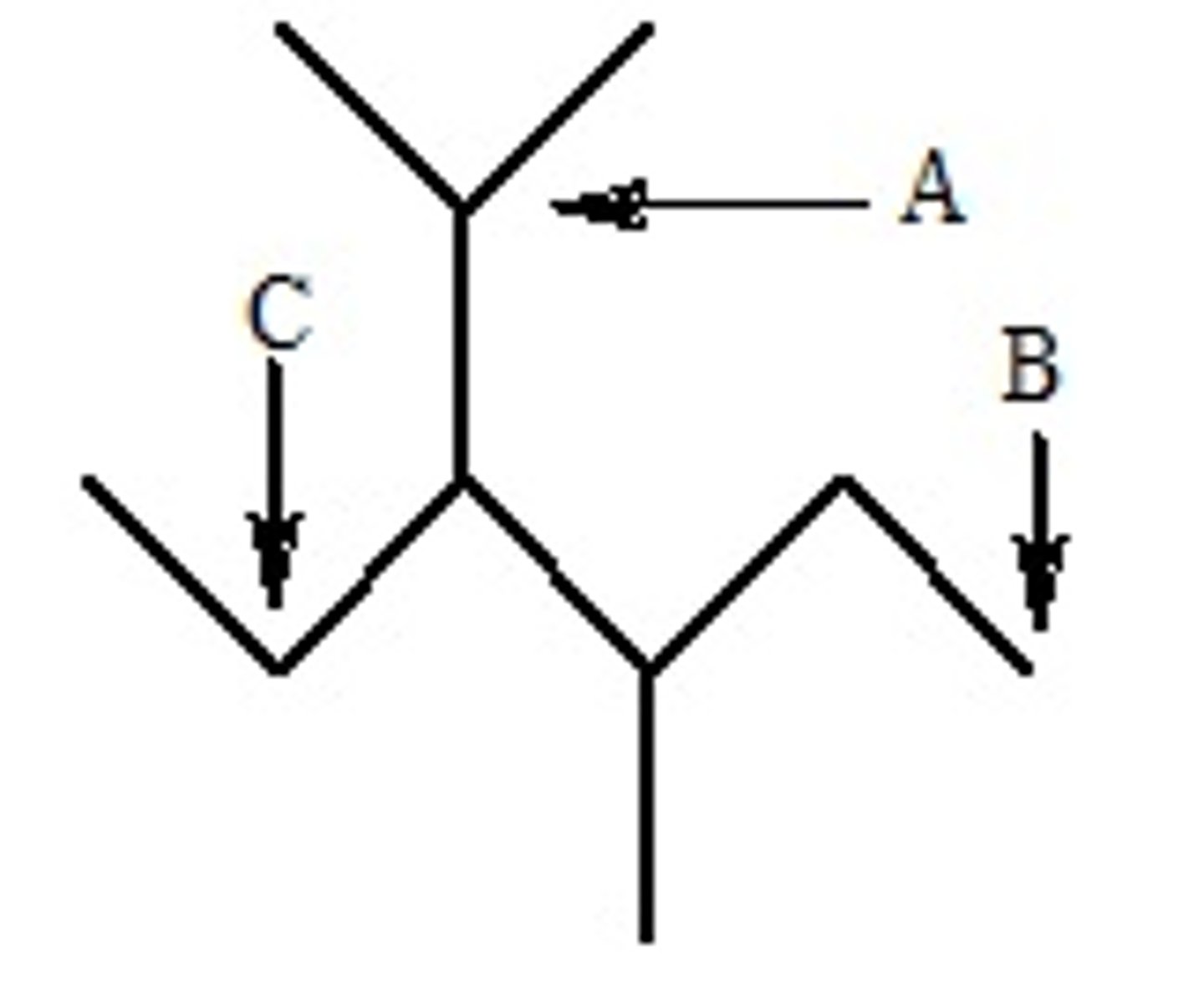
Three carbon atoms have been labeled on the molecule below. Which of these carbon atoms are tertiary?