Ch. 5a Notes ( AP Biology)
1/10
Earn XP
Description and Tags
Macromolecules
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Monomer
A monomer is a molecule that can be bonded to another identical molecule to form a polymer.
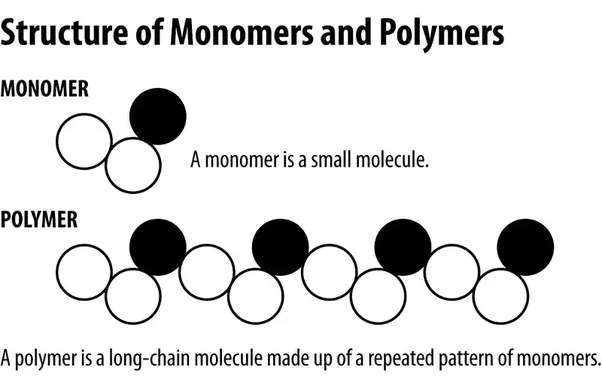
Polymer
A long chain of conjoined monomers that are used to make plastic
Condensation reaction
A chemical reaction where two smaller molecules combine to form a larger molecule, often releasing a smaller molecule of water
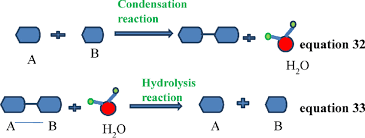
Hydrolysis
Where a molecule is broken down by the addition of water
Role of Carbohydrates?
Causes energy to build in the muscles and brain
What are the three properties used to classify amino acids?
Non-polar (hydrophobic)
Polar (Water soluble)
Charged (can form ionic bonds)
What is the characteristic common to lipids?
They are usually hydrophobic.
What are two differences between saturated and unsaturated fats?
Saturated: has a straight hydrocarbic link with a single bond
Unsaturated: Has a double bond that is weaker due to loosely packed acid chains.
How do phospholipids react in an aqueous solution?
They are non-polar and hydrophobic which will likely repel water in a solution.
What are three properties used to classify amino acids?
Non-polar, Polar and charged
What happens to a protein during denaturation?
It alters a proteins three dimensional structure which causes it to lose its characteristics.