Neurotransmitters/receptors
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
What is a neurotransmitter?
→ a chemical substance which is released at the end of a nerve fibre by the arrival of a nerve impulses, and by diffusing across the synapse, effects the transfer or the impulse to another nerve/muscle
usually a neuron will only submit one type of NT e.g. glutamatergic…
Effects on neurotransmission
Excitatory → increases likelihood of generating and action potential in the post-synaptic neuron
Inhibitory → decreases the likelihood
Neuromodulators
→ substance or device that alters nerve activity by affecting synaptic transmission
unlike NTs, neuromodulators don’t directly transmit signals across a synapse, but influence the strength and response of neurons
E.g. hormones, amino acids
Traditional ‘small’ molecule NTs
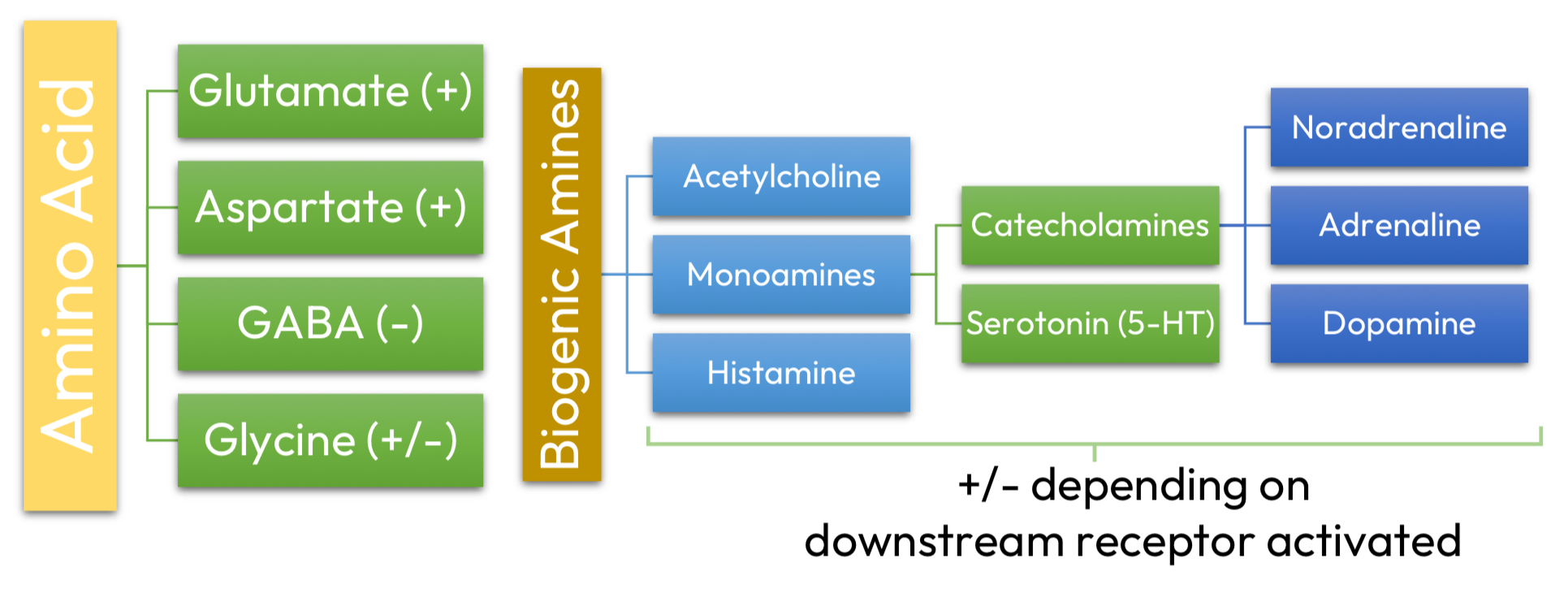
Chemical mediators in the CNS
Traditional ‘small’ molecule NTs = fast/slow synaptic transmission, neuromodulation too
Neuroleptics = neuromodulators
Lipid mediators = neuromodulators
Nitric oxide = neuromodulators
Neurotrophins, cytokines = neuronal growth/survival
Steroids = functional plasticity
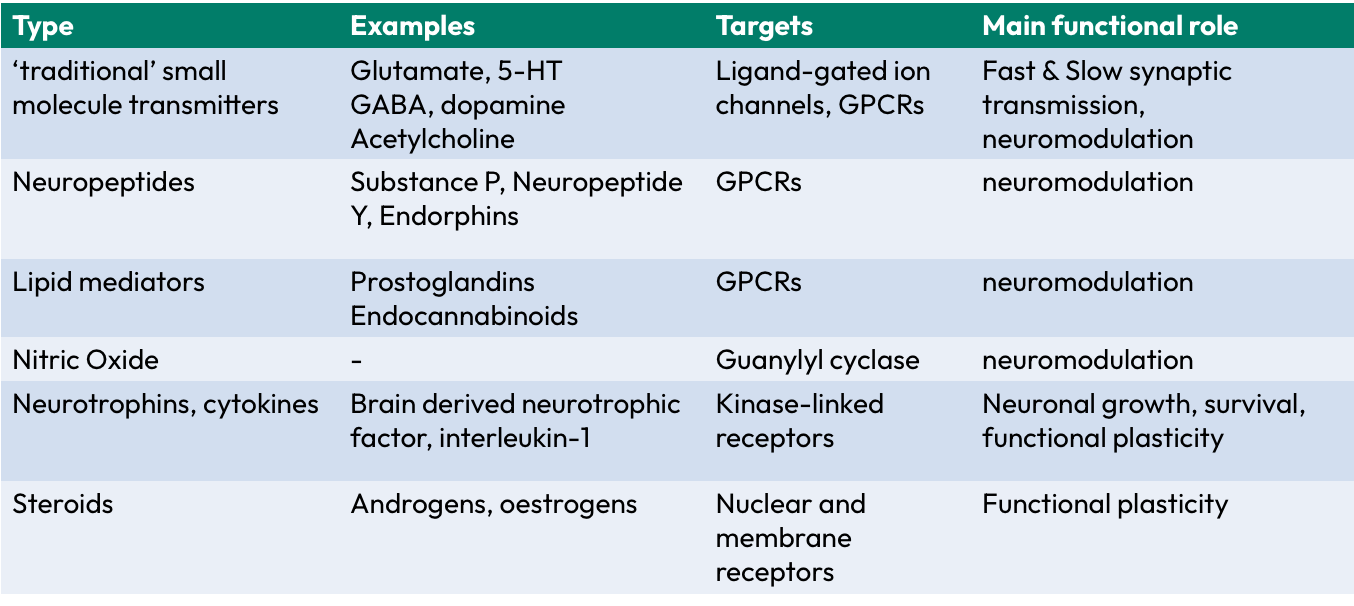
Glutamate
main excitatory NT in the brain
>90% of synaptic connections in the brain
Ionotropic receptors = iGluRs (AMPA, NMDA, Kainate)
Metabotropic receptors = mGluRs (grp 1, grp 2…)
Fast NT
GABA
Gamma-amino butyric acid
main inhibitory NT in the CNS
Fast NT
Ionotropic receptors = iGABARs (GABAa, GABAc)
Metabotropic receptors = GABAb receptors
Ionotropic Glutamate receptors
AMPA, NMDA, Kainate
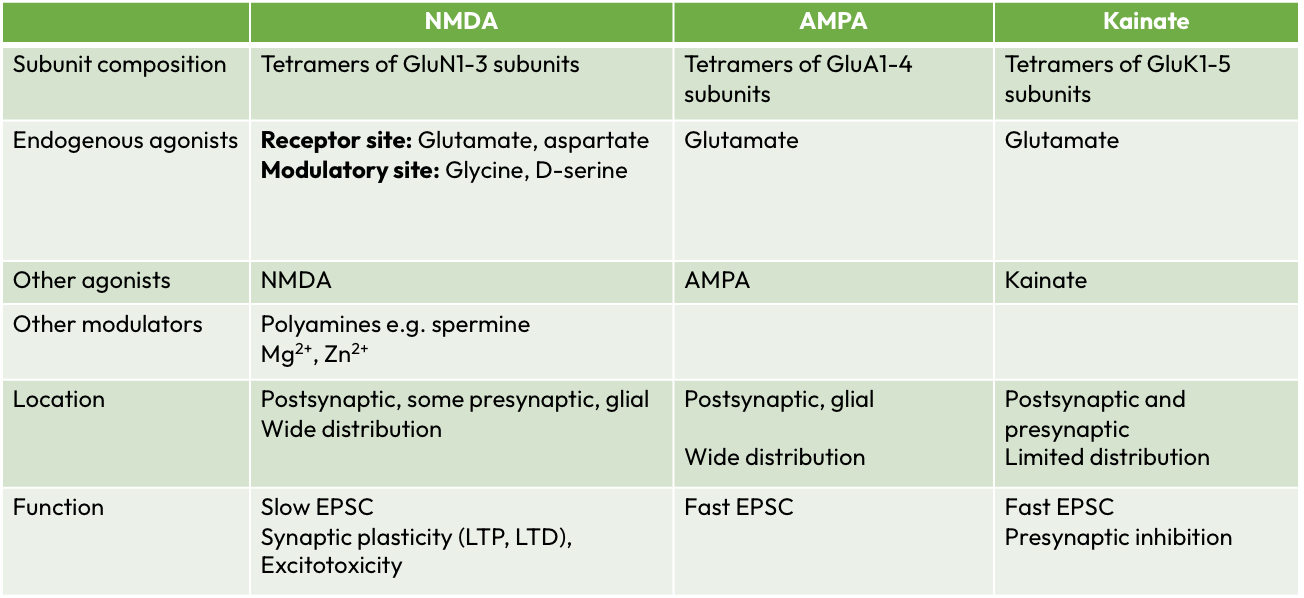
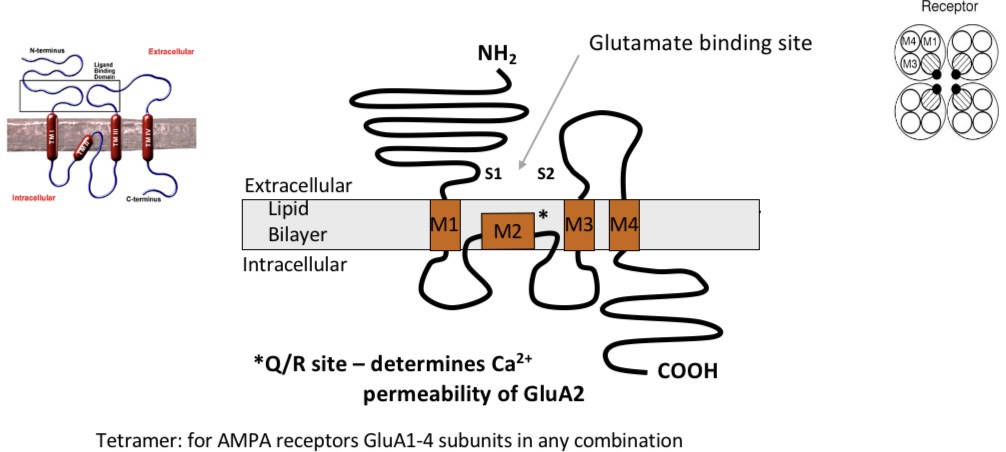
AMPA receptors
Properties:
mediate majority of fast excitatory transmission
Non-selective cation (+ve) channels permeable to Na+ (in) and K+ (out)
4 subunits to form a tetrameric receptor
GluA1-4, which mix and match to produce subtly different receptors
Those containing GluA2 subunits have very low Ca2+ permeability
Activation of all AMPA receptors leads to an influx of Na+, but receptors are only permeable to Ca2+ in Abscence of GluA2
Kainate receptors
GluK4 and 5 don’t form function receptors alone, must be both
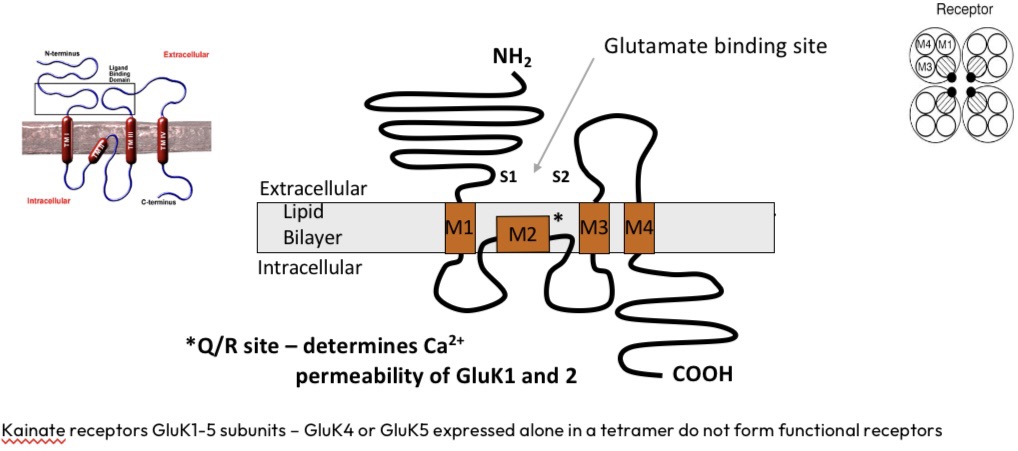
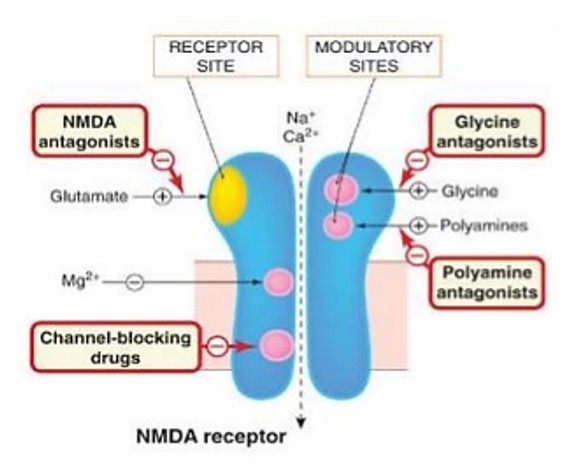
NMDA receptors
cation channel for Na+ and Ca2+
Channel opening = depolarisation
Acriviation requires binding of a glutamate (orthosteric site) and a co-agonist (modulatory site)
Has a voltage sensitive Mg2+ block which is present usually, but removed when cell depolarises
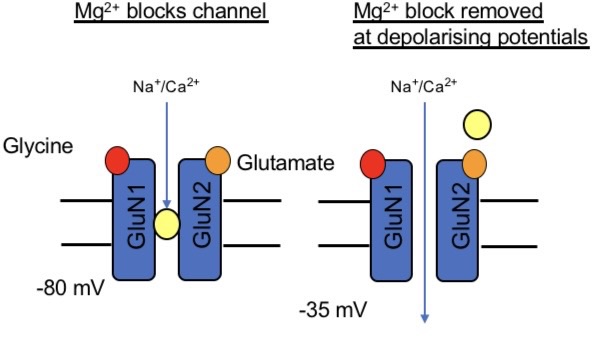
Mg2+ block
in order for block or NMDAR to occur, the channel must be open e.g. glycerine and glutamate bound
As membrane potential is depolarised, the block os progressively removed
Coincidence detector:
AMPARs often present at the same synapses as NMDARs
Activation of AMPARs depolarises the membrane sufficiently to remove Mg2+ block
Ca2+ entry through NMDARs is dependant on pre/postsynaptic elements being activate at the same time
NMDAR is the coincidence detector
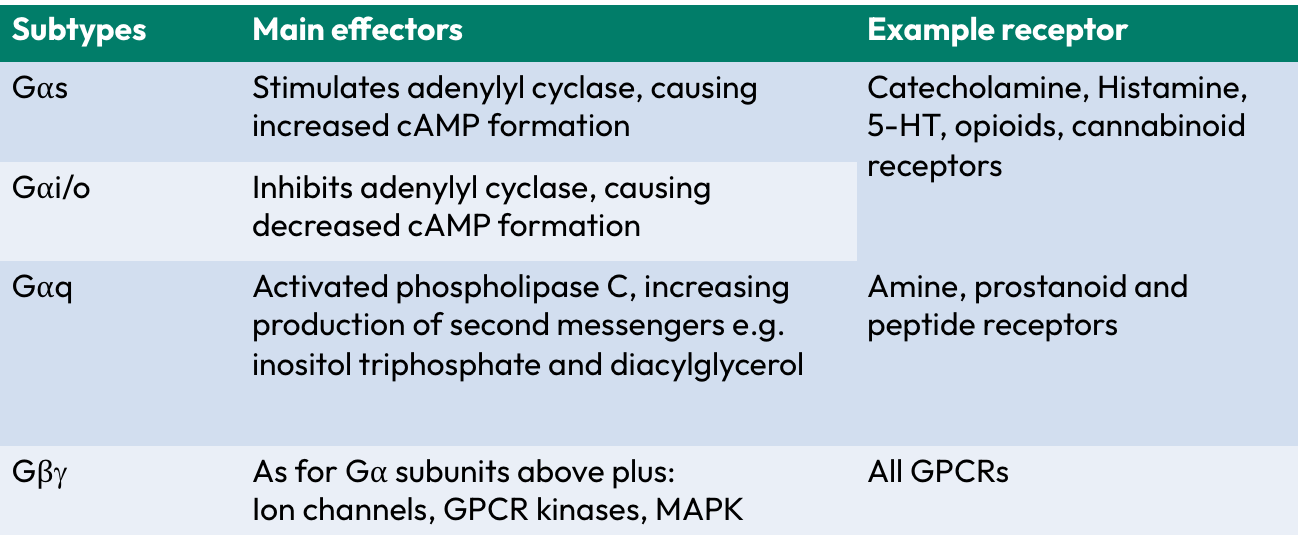
Main g-protein subtypes
Gas
Gai/o
Gaq
Gby
Metabotropic glutamate receptor subunits
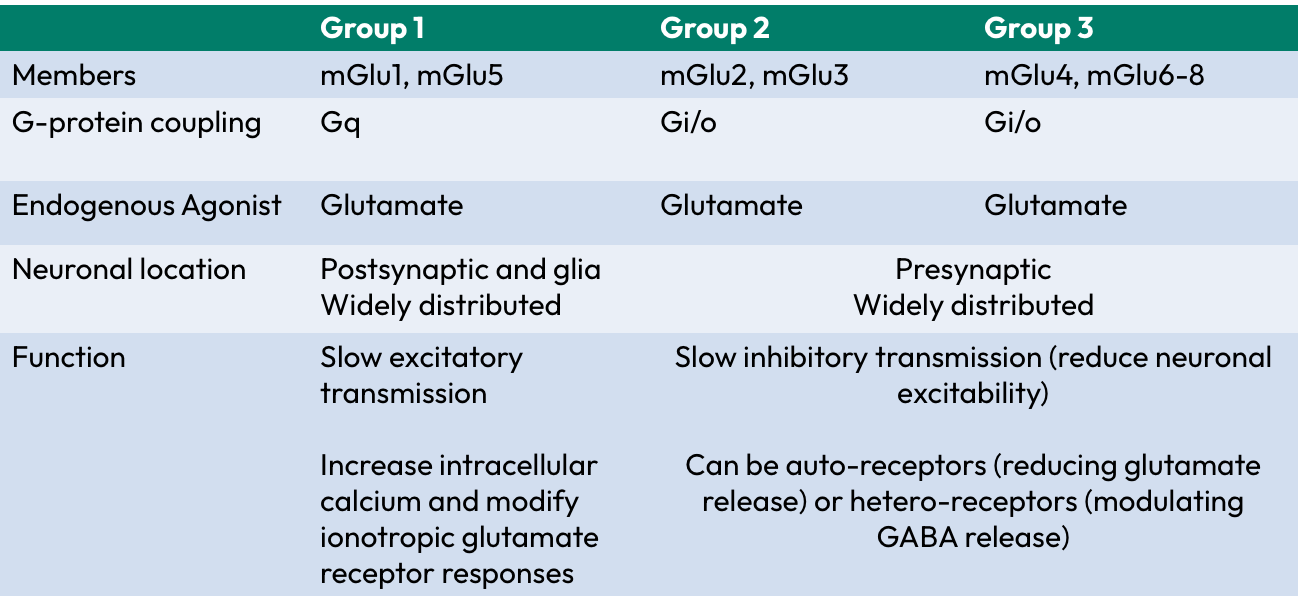
Structure of mGlu receptors
bi-lobed N-terminal contains glutamate binding site
Cysteine rich domain involved in maintaining tertiary structure
7 transmembrane domains
Second intracellular loop involved in G-protein coupling and determining transduction mechanism
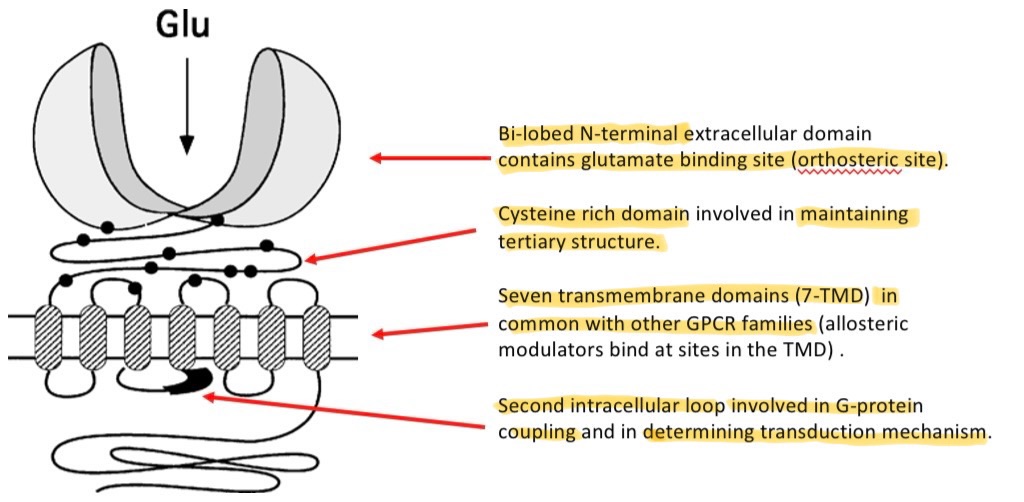
Properties of mGlu receptors
depending on subtype, can be pre/postsynaptic elements being synaptically localised
Play a modulatory role in synaptic trasnmission
Post-synaptic group 1 receptors mediate slow depolarisation (EPSPs)
Presynaptic groups 2 & 3 decrease NT release
Desirable targets fro drug discovery
Modulation of signalling through K+ and Ca2+ → control of excitability of neurons
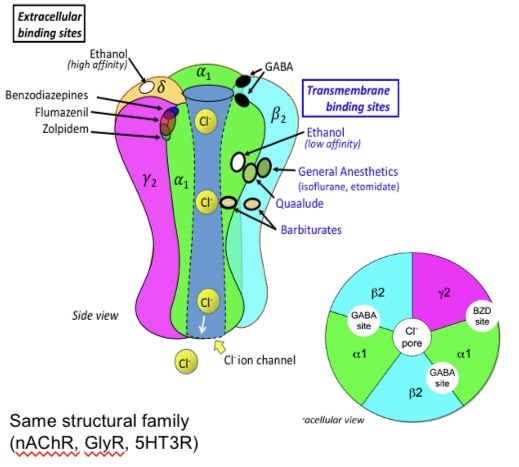
GABAa receptors
anion channel (cL-). Channel open = hyperpolarisation
5 subunits- 2 alpha, 2 beta, gamma
Isomers have different sensitivity to alcohol
Found synaptically (short term) and extra-synaptically (modulating tone of neural circuits)
Sedative/hypnotic drugs enhance GABAa receptor activity via the modulatory site
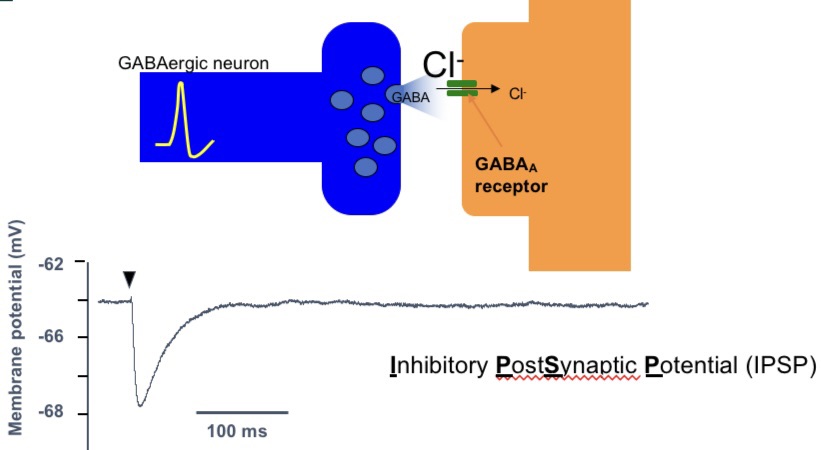
GABAergic synaptic transmission (GABAa)
synaptic transmission = fast
CL- detected by GABAa creates an IPSP (hyperpolarisation)
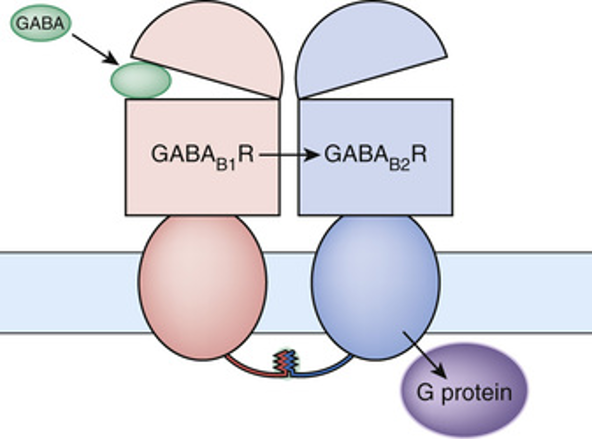
GABAb receptors
dimer made of two 7 transmembrane domains Second intracellular subunits held together by coil interactions between C-terminus tails
Activation occurs when GABA binds to extracellular domain of B1 subunit
Located pre and postsynaptically
GPCR that couples through Gi/Go
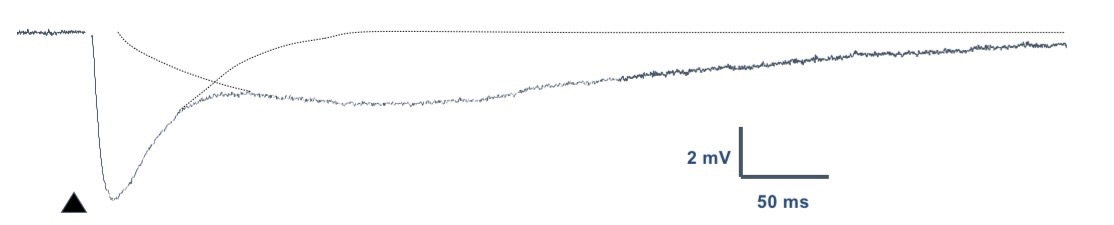
Coactivation of GABAa and GABAb
produces long-lasting biphasic IPSPs
Synchronous release of GABA results in the simultaneous activation of both receptors
Neuromodulation: alcohol
alcohol affects neural function, full spectrum of effects is unclear currently
Can modulate glutamatergic neurotransmission:
non-competitive antagonist (-ve allosteric modulator) → NMDA and AMPA receptors (Ionotropic) decreasing effect
Reduced glutamate release from presynaptic terminal → increases activity of mGluR2/3
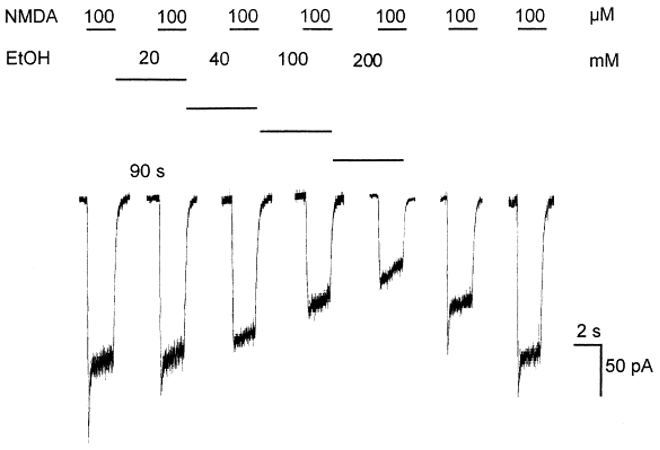
Ethanol effects on NMDA currents
→ ethanol inhibits NMDA mediated currents
Ethanol dose dependently reversibly inhibits NMDA-induced inwards currents in cultured neurons (voltage clamp)
Other studies indicate ethanol is a non-competitive antagonist
Different glutamate receptor subunits have different ethanol sensitivity, which explains why areas of the brain are affected differently to alcohol
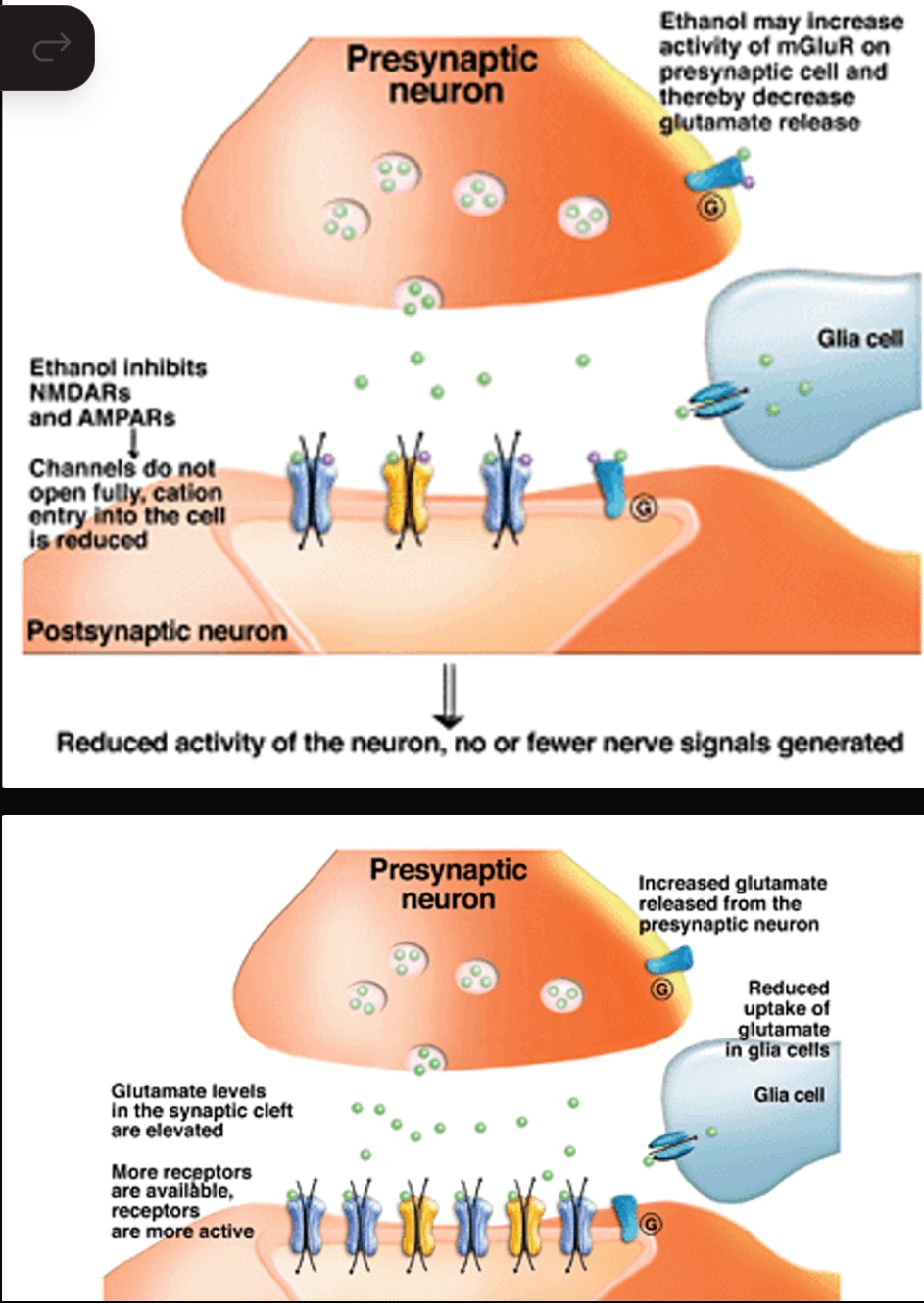
Alcohol effects on glutamate transmission
acutely inhibits glutamate neurotransmission
Reduced activity of the neuron, no/fewer nerve signals generated
Chronic alcohol intake leads to compensatory adaptations in neurotransmission of glutamate = excessive activity of the neuron
Behavioural effects of alcohol
→ mediated by changes in glutamatergic signalling
Acute (reduced signalling) = amnesia, memory loss (intact NMDA signalling required for memory formation)
Chronic/withdrawal (enhanced signalling) = seizures/brain damage/excitotoxicity, anxiety, disorientation (hyper excitability associated with withdrawal)
Foetal alcohol syndrome
maternal alcohol levels impair glutamatergic signalling in the developing brain
Reduced NMDA receptors in offspring
Developmental and cognitive impairment
Alcohol modulating GABAergic transmission
ethanol modulates GAABAergic neurotransmission
Positive allosteric modulator = enhances Cl- influx through GABAa receptors
Enhanced GABA release = acting via presynaptic GABAb receptors
Chronic exposure to alcohol: GABAergic transmission
chronic exposure leads to a recused impact on GABAergic transmission
Change in GABAa receptor subunits composition
Reduced sensitivity of GABAa to alcohol/neurosteriods
No change in receptor number
Behavioural effects of alcohol: GABA
→ alcohol mediates chances in GABAergic signalling
Acute (reduced signalling) = sedative, anxiety-reducing, impaired coordination
Chronic/withdrawal (enhanced signalling) = alcohol tolerance (changed subunit composition) and seizures/tremor (hyper excitability due to loss of inhibitory tone)