ORGO 2, Test 2 Things I don't know
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
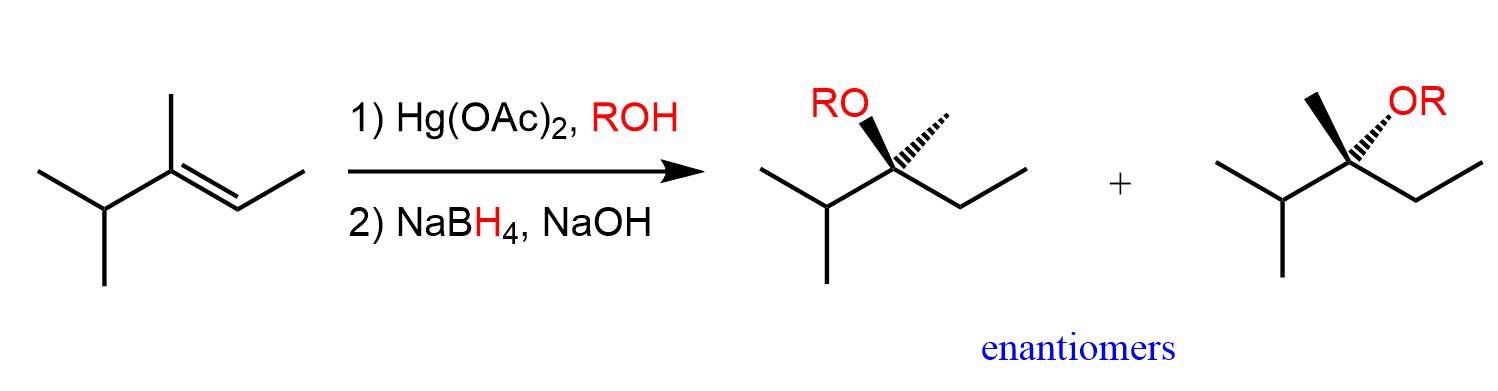
CH 18: Alkoxymercuration/demercuration for addition of ROH
Concerted:
Pericyclic:
Sigmatropic:
Concerted – all bond breaking happens at same time (diels alder)
Pericyclic – transition state involves cyclic redistribution
Sigmatropic – sigma bonds migrate across pi system ex: Claisen
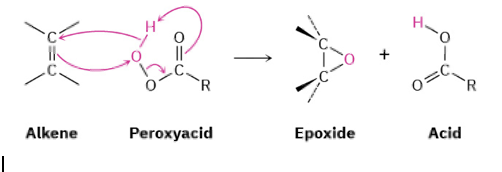
alkene with peroxyacid
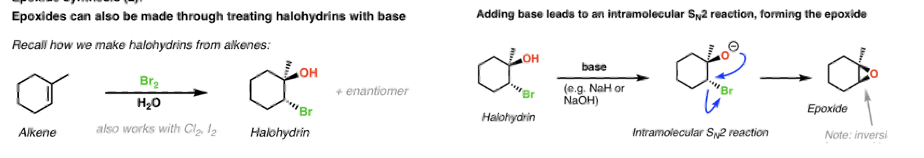
Vicinal halohydrin in base to form epoxide (alkene)
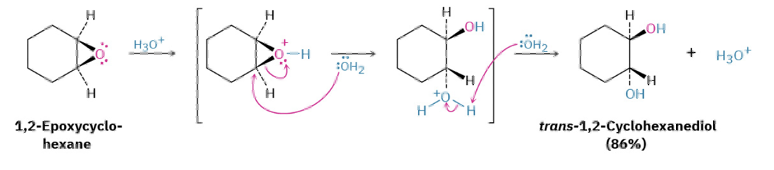
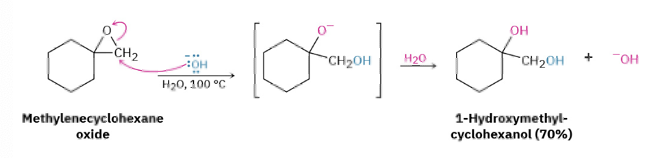
Ring opening for epoxide with Acid vs Base
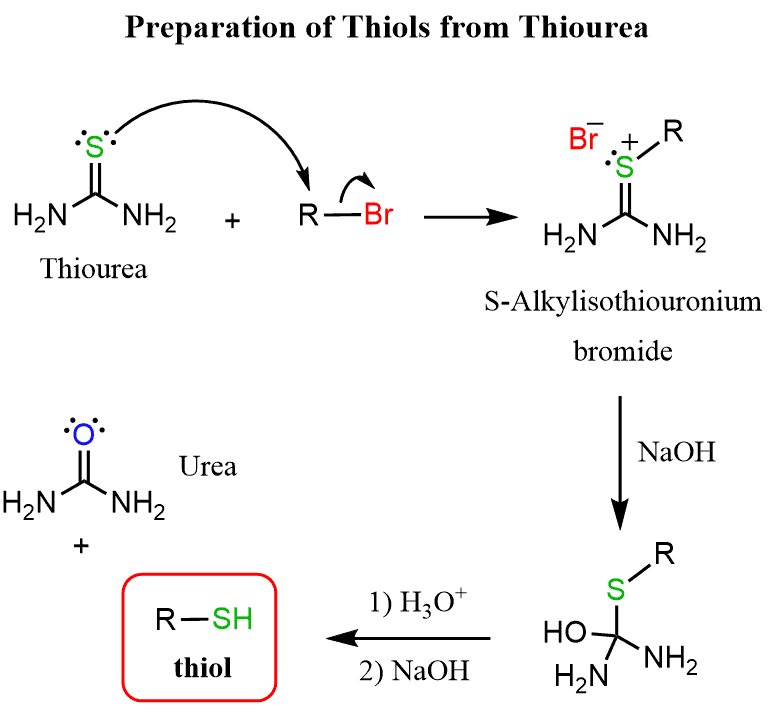
Sythesis of thiols by alkylation and hydrolysis of thiourea (halide)
Oxidation of thiols to disulfides and reverse reaction
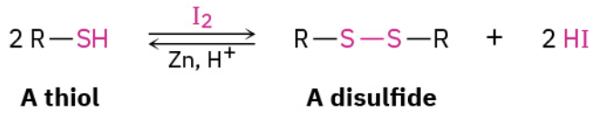
sythesis of aldehydes from primary alcohols
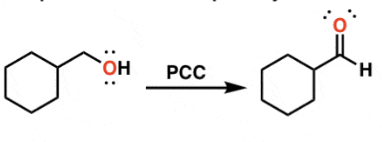
PCC, PDC, DMP
Syhthesis of ketone from alkene
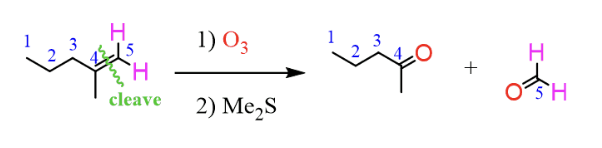
O3
Synthesis of aldehyde from ester
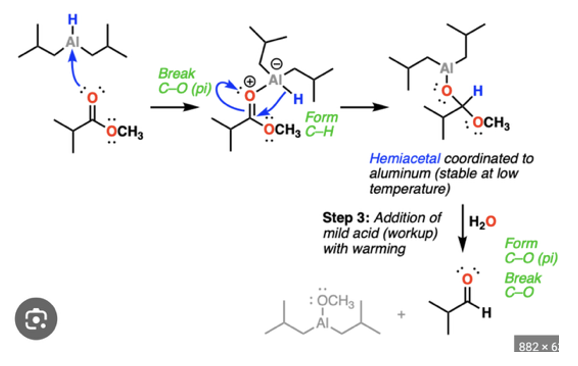
DIBAL orrrr,1)LiAl4 2) H2O —> [O]
Ketones by alkyne
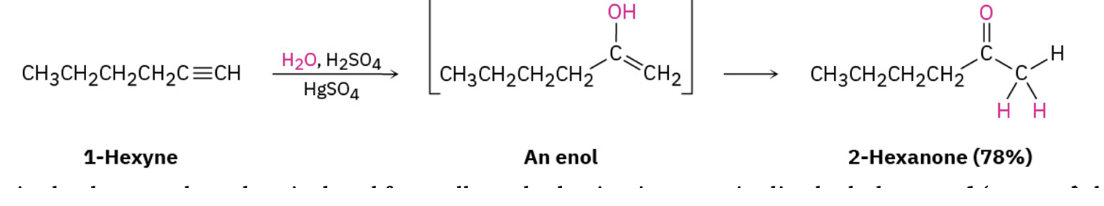
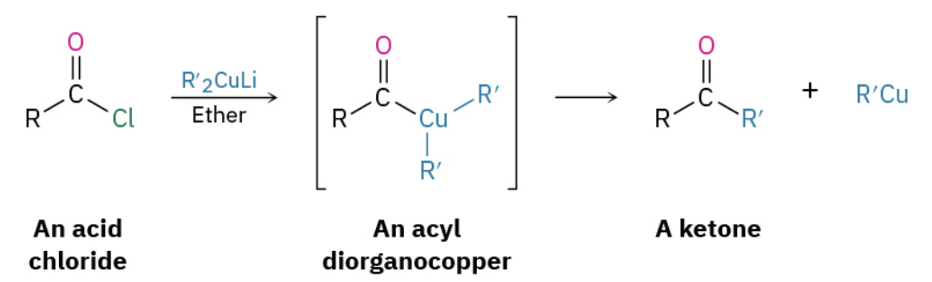
Ketone synthesis by organocooperate
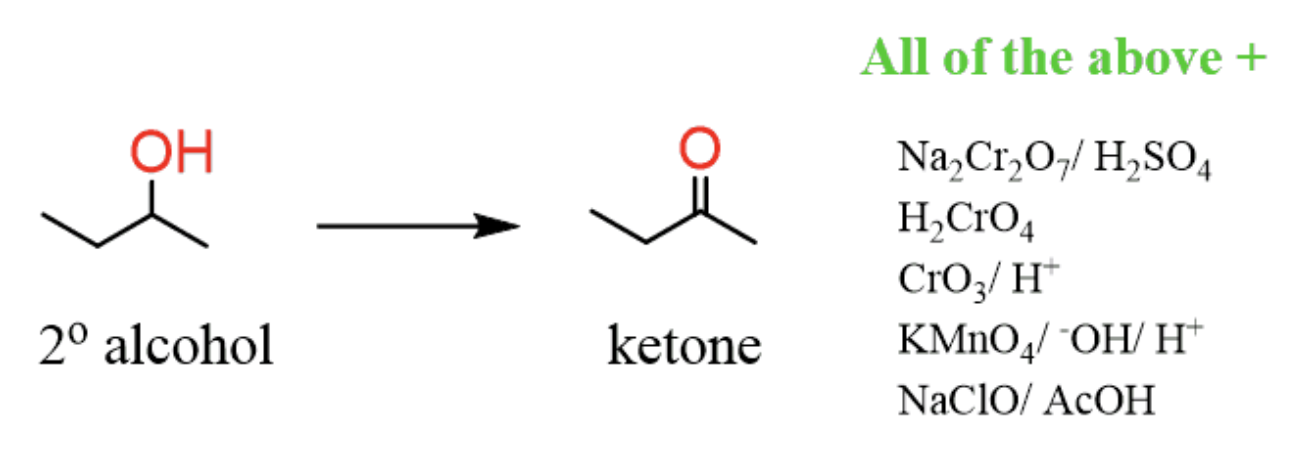
Synth Ketones by oxidation of secondary alc
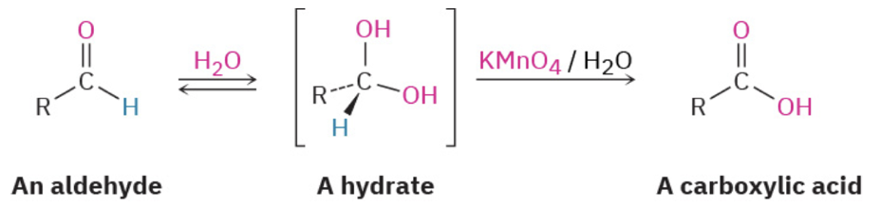
(Not mechanism) oxidation aldehyde
What are:
imines
cyanohydrins
enamines
hydrates
hemiacetals
acetals
hydrazones
oximes
phorphorus ylids
R=NR
OHRCN
R=CNR2
R(OH)2
R(OH)(OR)
R(OR)2
R=NNH2
R=NOH
PPh3
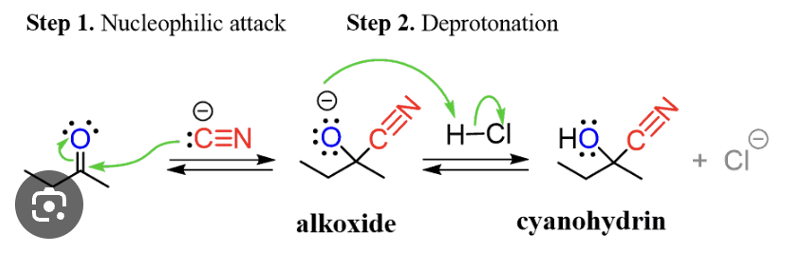
structure and mech of addition HCN to aldehyde or ketone
prod and mech formation of imines
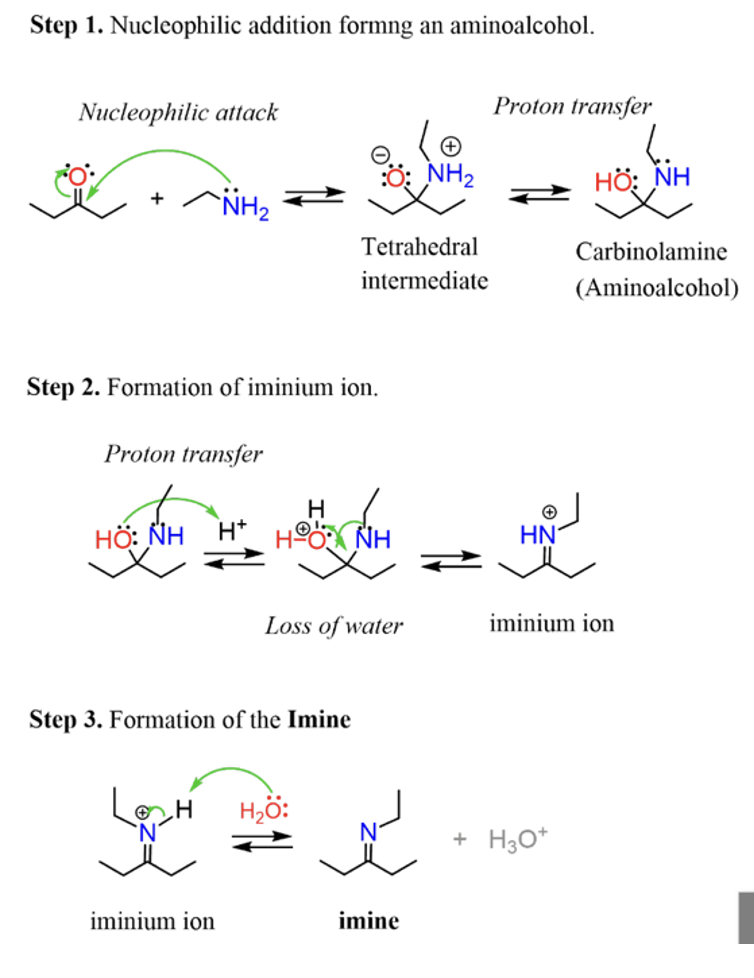
Hydrazine with ketone
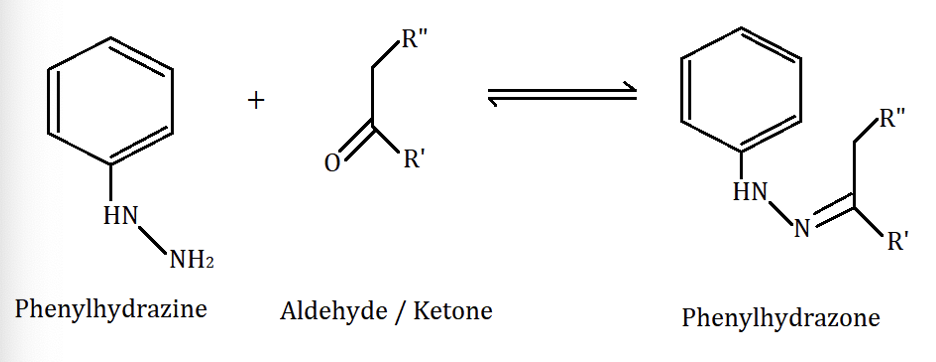
Phenylhydrazine in acid
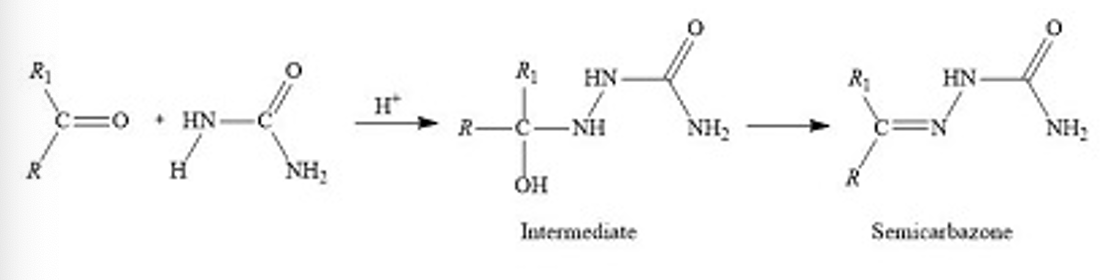
Semicarbazide synthesis
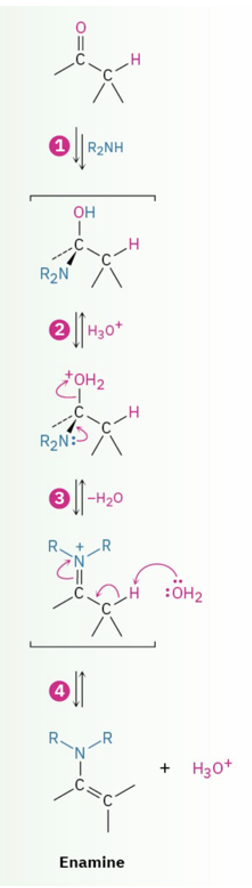
Formation of enamines
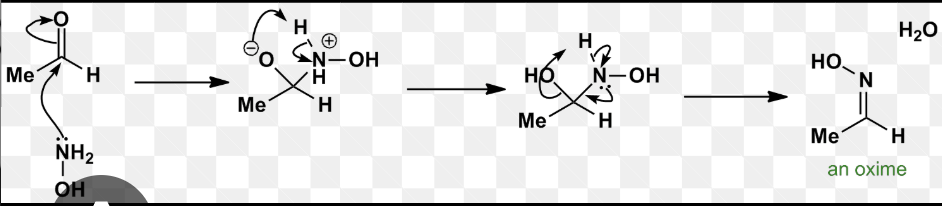
hydroxylamine with ketone/ald
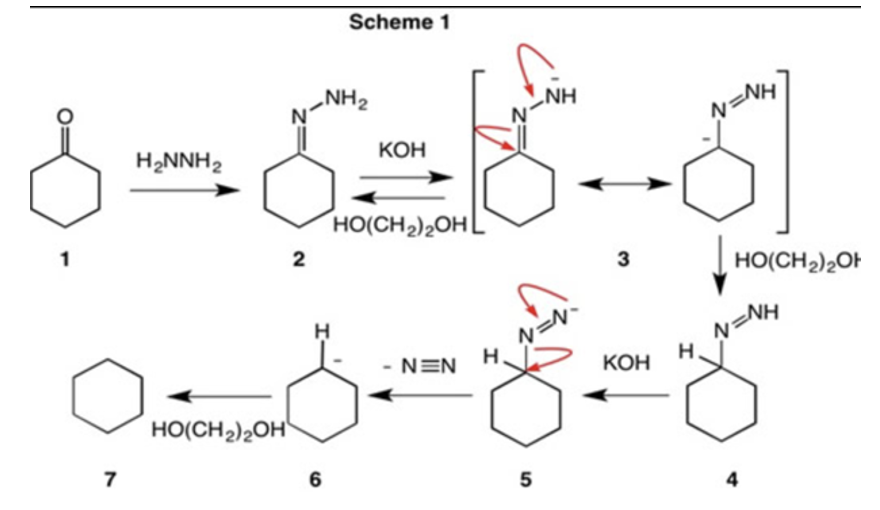
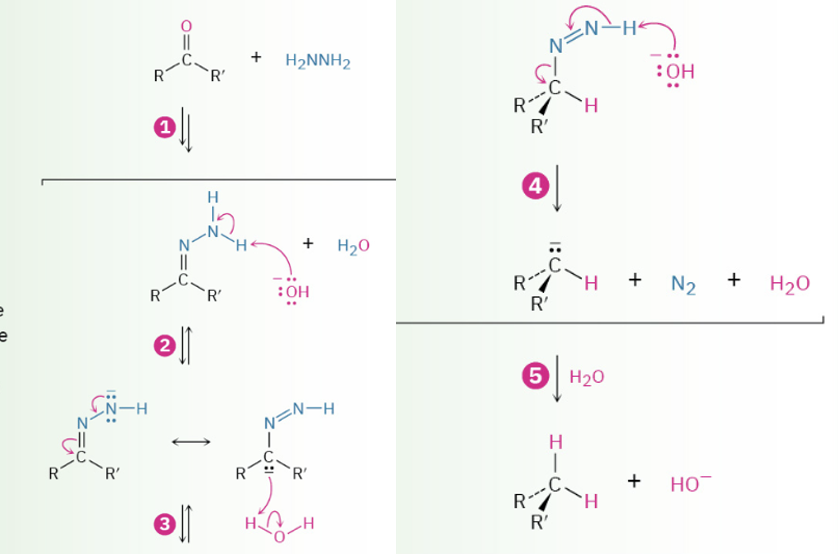
Wolff Kishner reduction
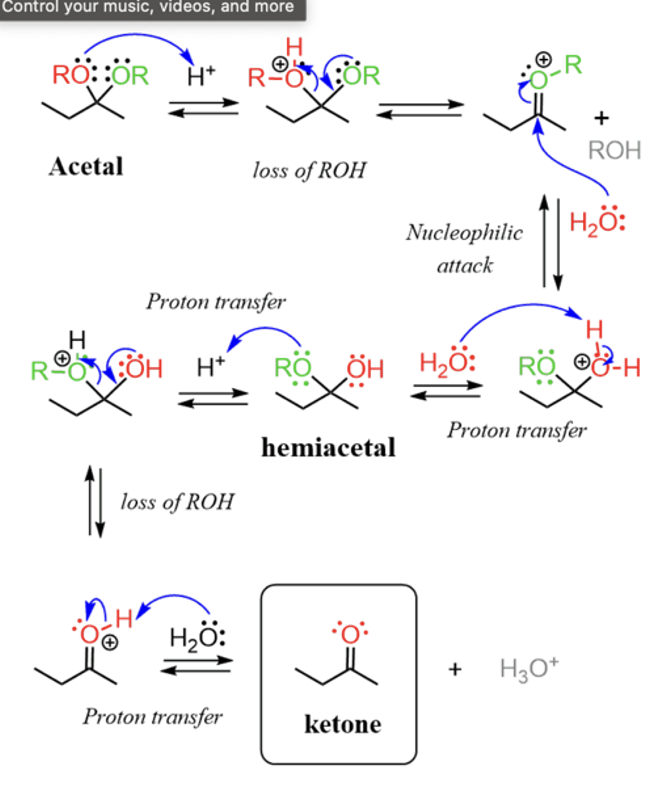
Acid catalyzed formation acetals
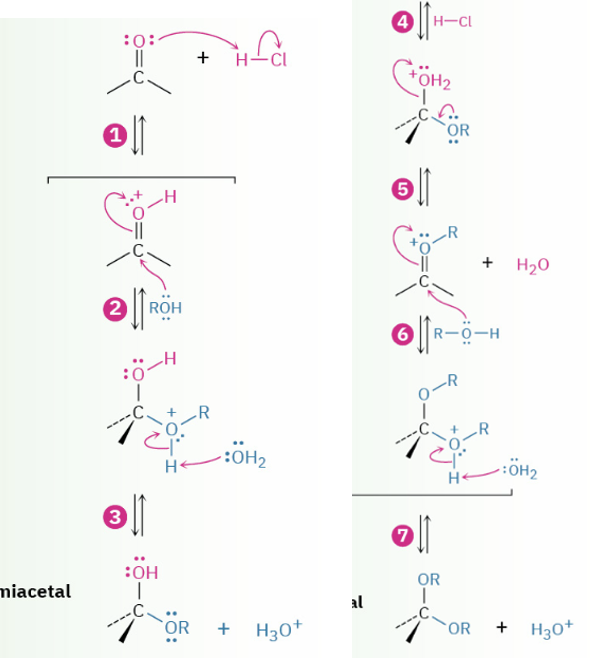
Conversion acetals to ketone or ald
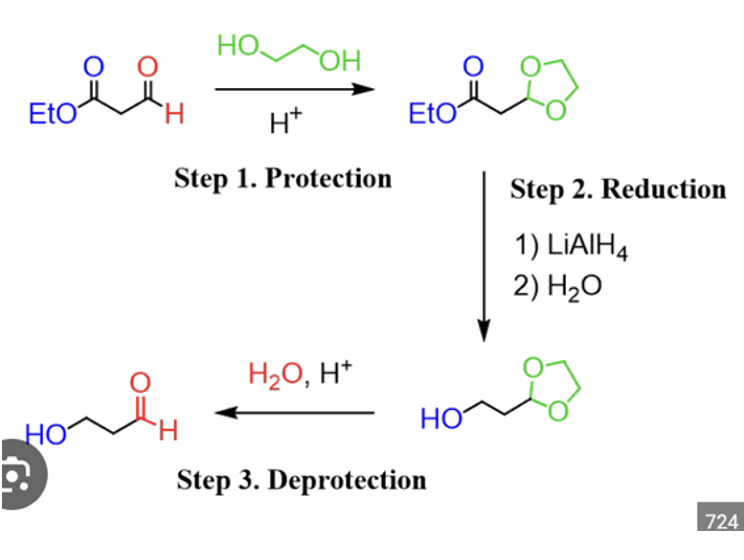
Acetals with protecting groups
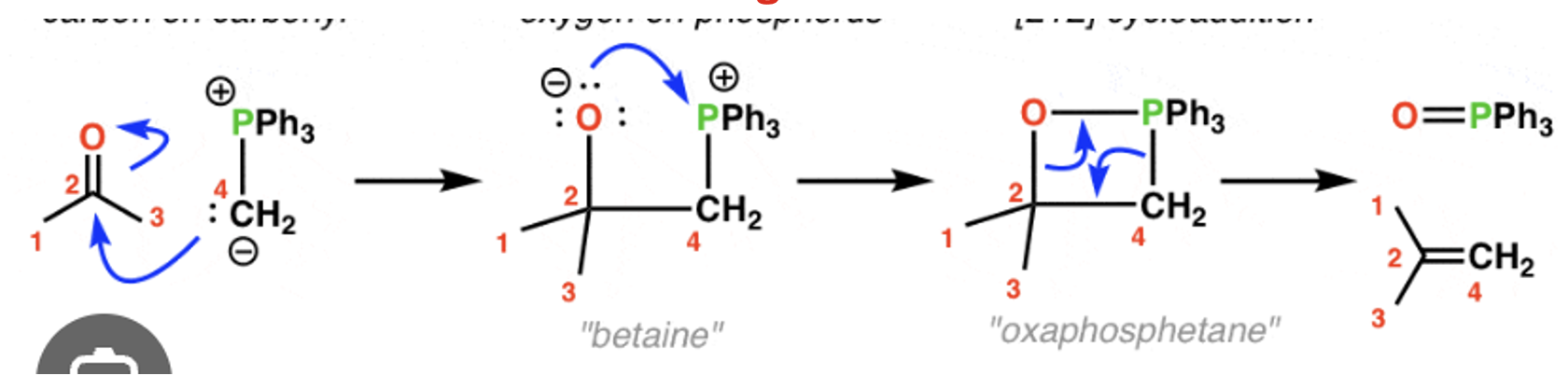
Wittig reaction
1,4 reacting nucleophiles
Amines (NH2)
CN-
RS-
Enolates (- charge O + C that resonate)
Orgaocuperates
enamines (—NH—)
1,2 reacting nucleophiles
Organolithium, Grignard reagents, organosodium, NaBH4, and LiAlH4 add to the C=O
PKa alcohol, phenol, carbox acid
16, 10, 5
Oxidation of aldehydes
Na2Cr2O7/H2SO4, KMnO4, CrO4, Tollens’ (Ag), Benedict Reagent (Cu) specifically for aldehyde to carboxylic acid, won’t do oxygen
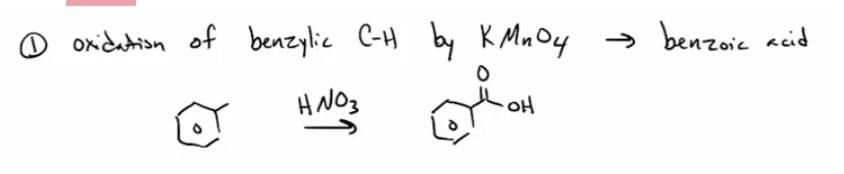
Oxidation of benzylic CH groups (
Formation carbox acid with grinyard or organosoduum (mechanism too)

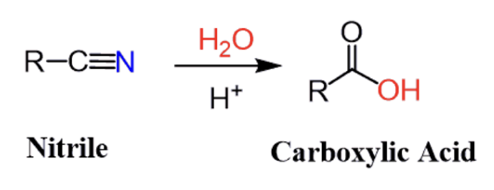
catalyzed hydrolysis of nitrile (mechansim too) (basic and acidic)
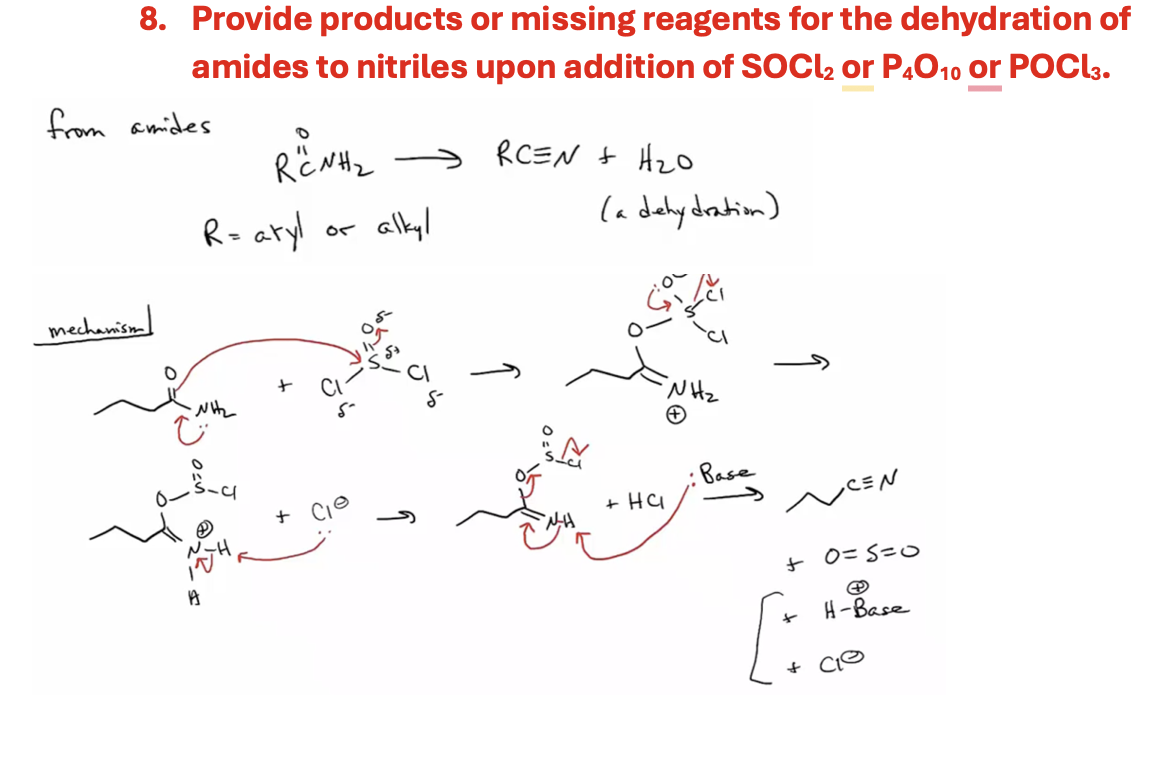
Products and reagents dehydration of amides
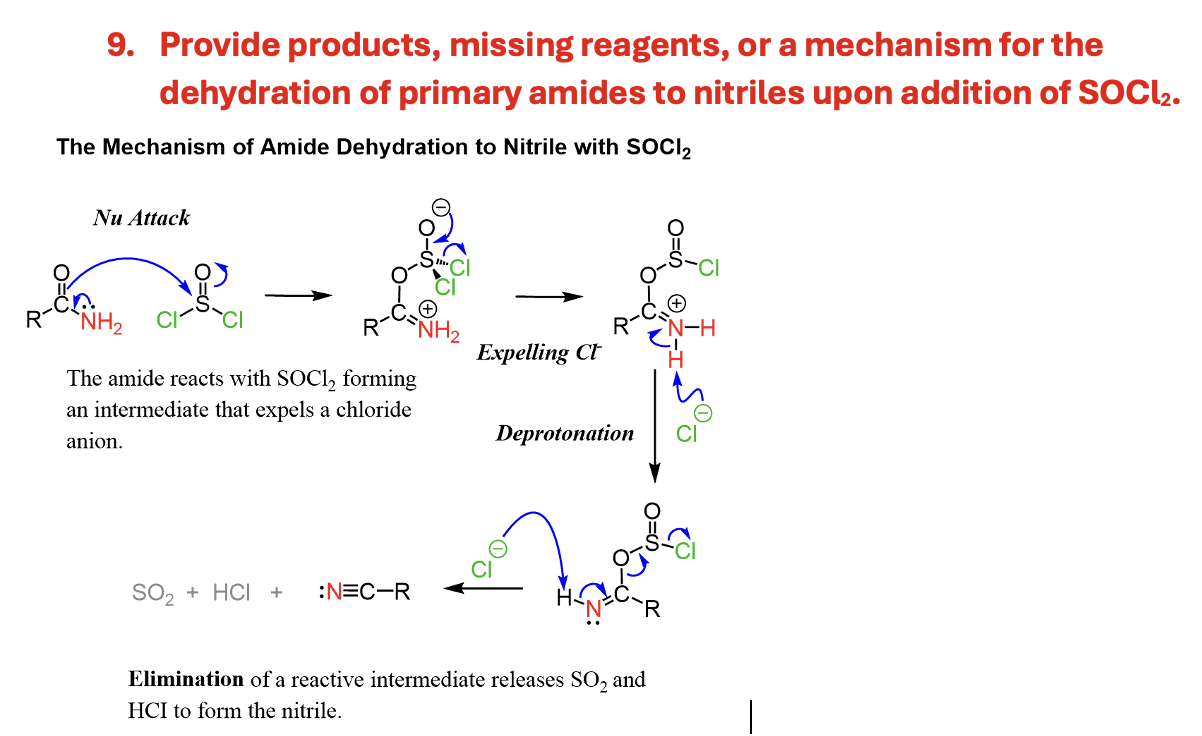
Provide products, missing reagents, or a mechanism for the dehydration of primary amides
Provide products and a mechanism for the reaction of a nitrile with a Grignard
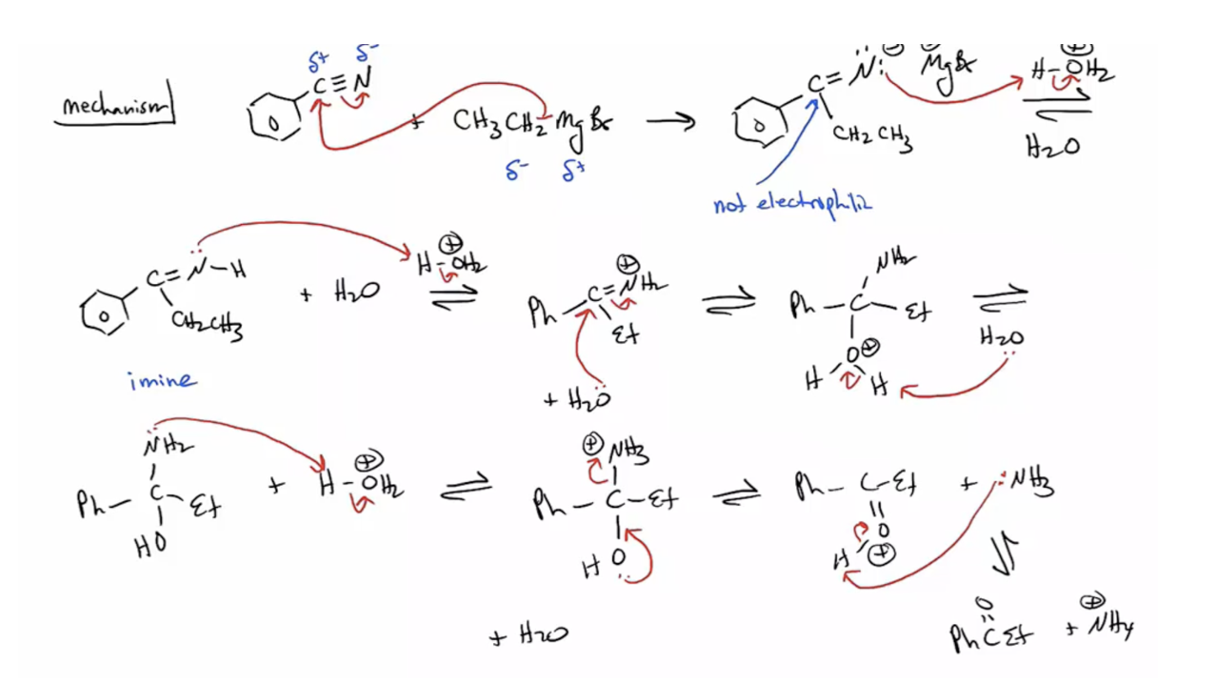
follow by aq acidic workup
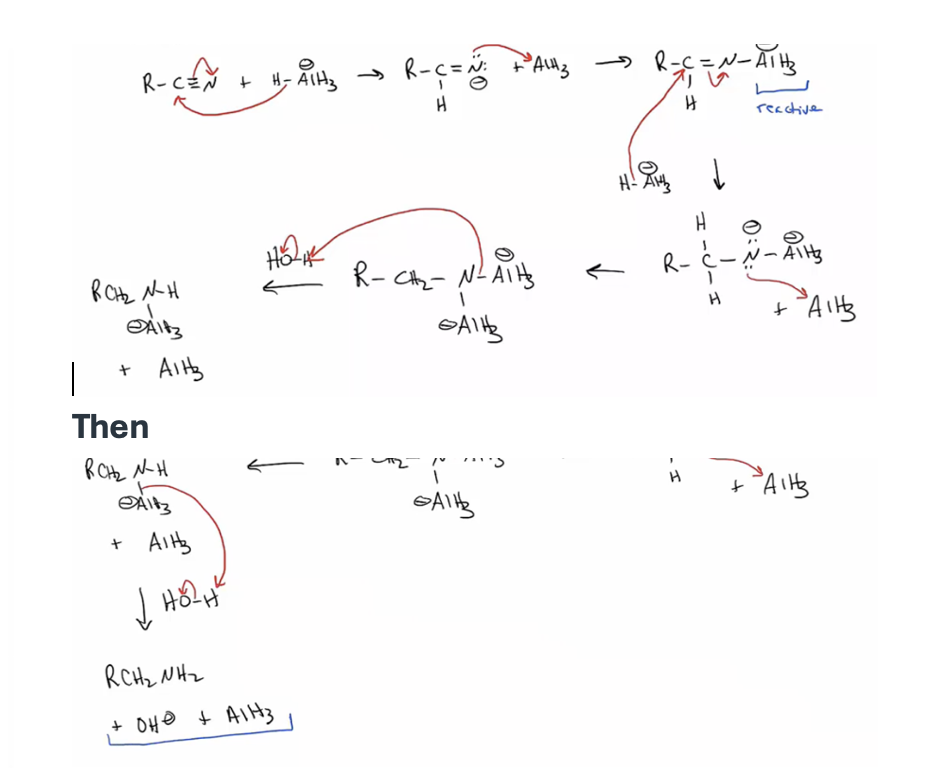
Provide products and a mechanism for the reduction of nitriles
primary amine with addition LiAlH4