EX2 Beta Antagonists (MC)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
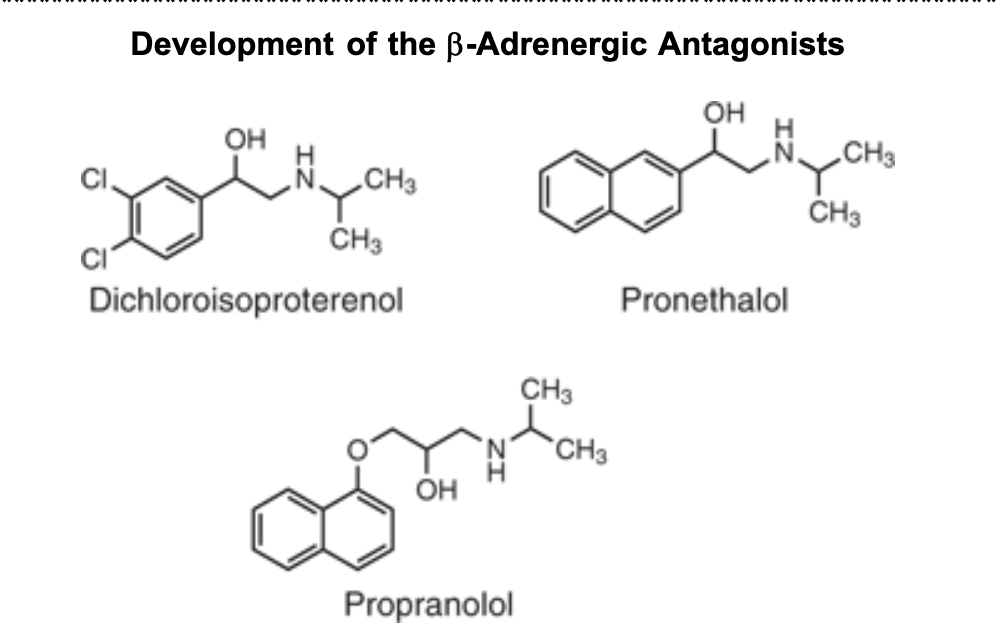
Development of the B-Adrenergic Antagonists
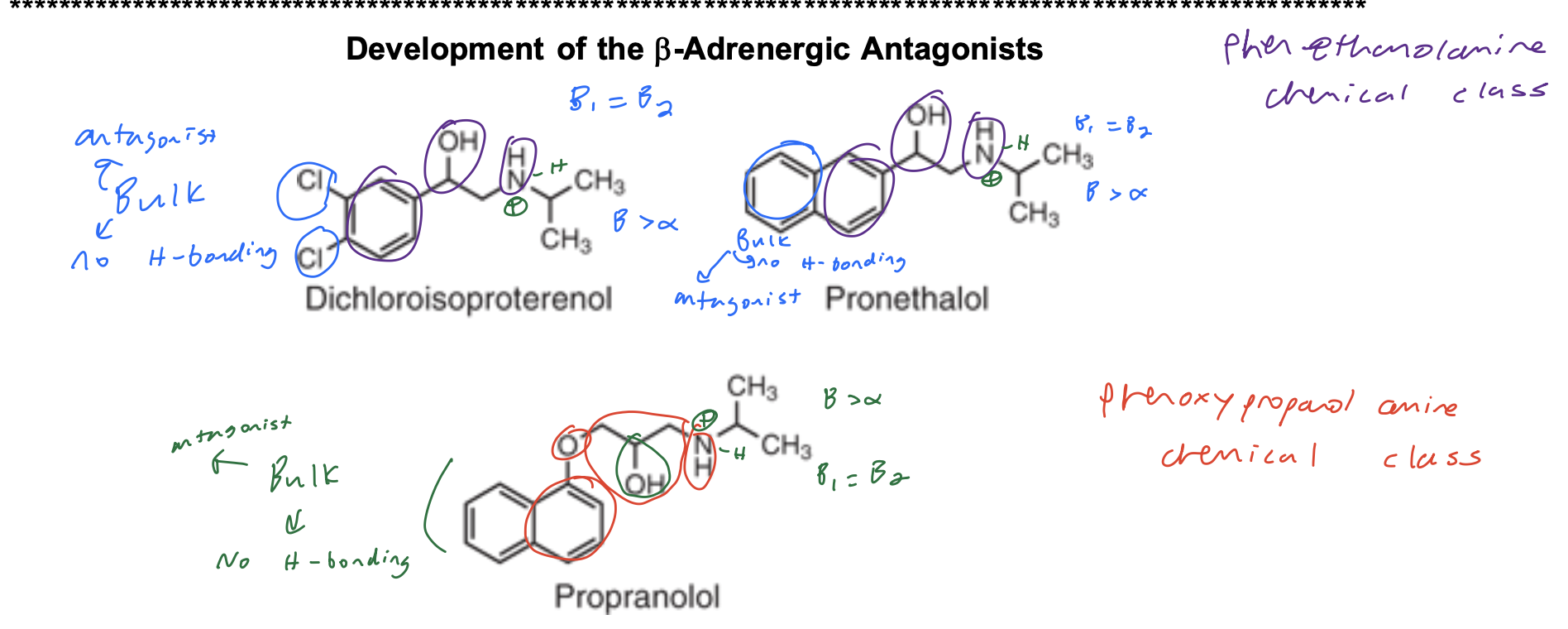
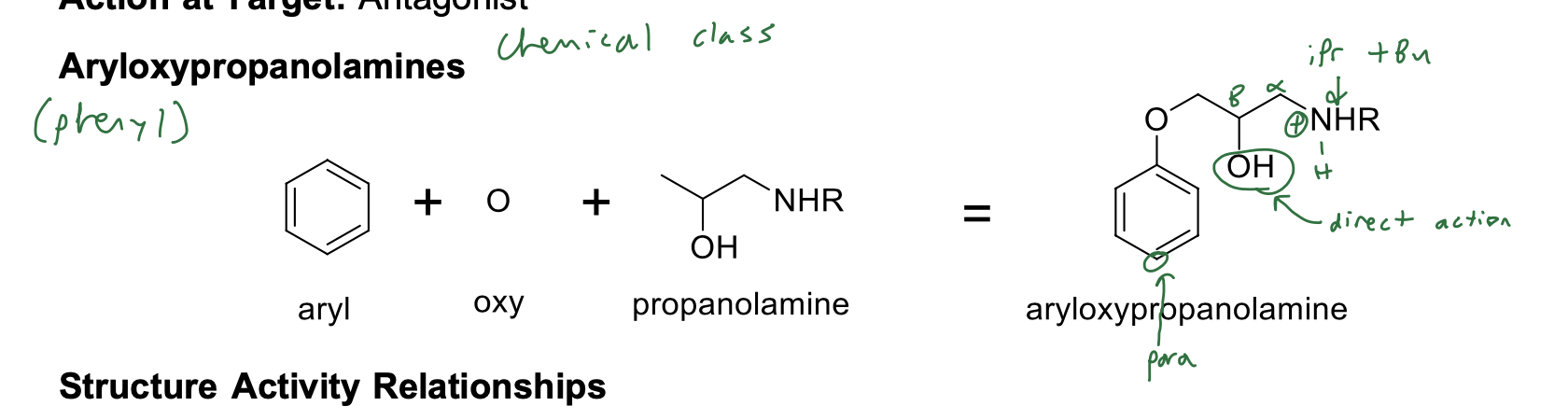
Aryloxypropanolamines (phenyl also works) SAR
Structure Activity Relationships
• Aryloxypropanolamine; NO substituent para to the oxygen on the aryl ring (B1=B2)
• Large branched alkyl group on the basic nitrogen (isopropyl or t-butyl)
• Basic nitrogen is required for binding; ionized form binds to receptor
• NO H-bond donor/acceptors directly attached to the aryl ring (except pindolol and carteolol) (no catechol, no catechol mimics)
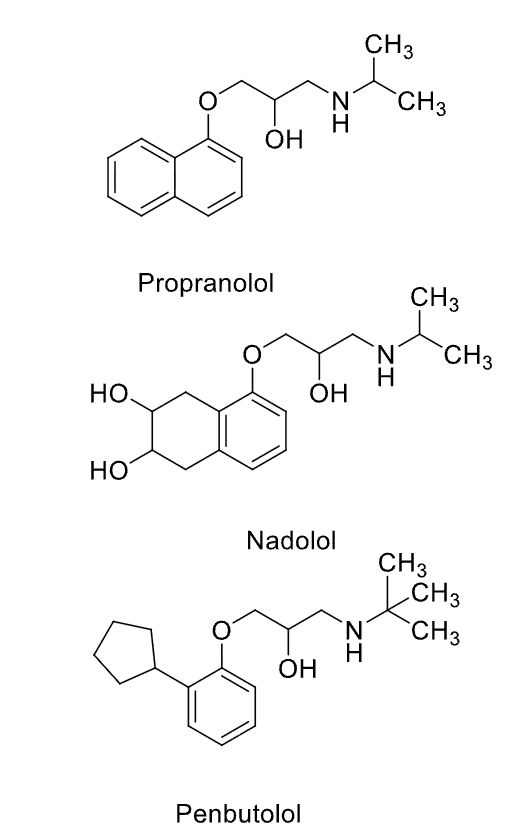
Aryloxypropanolamines examples

Non-selective B antagonists with Intrinsic Sympathomimetic Activity (ISA)
Look for an H-bond donor/acceptor N-H group directly attached to the aromatic ring
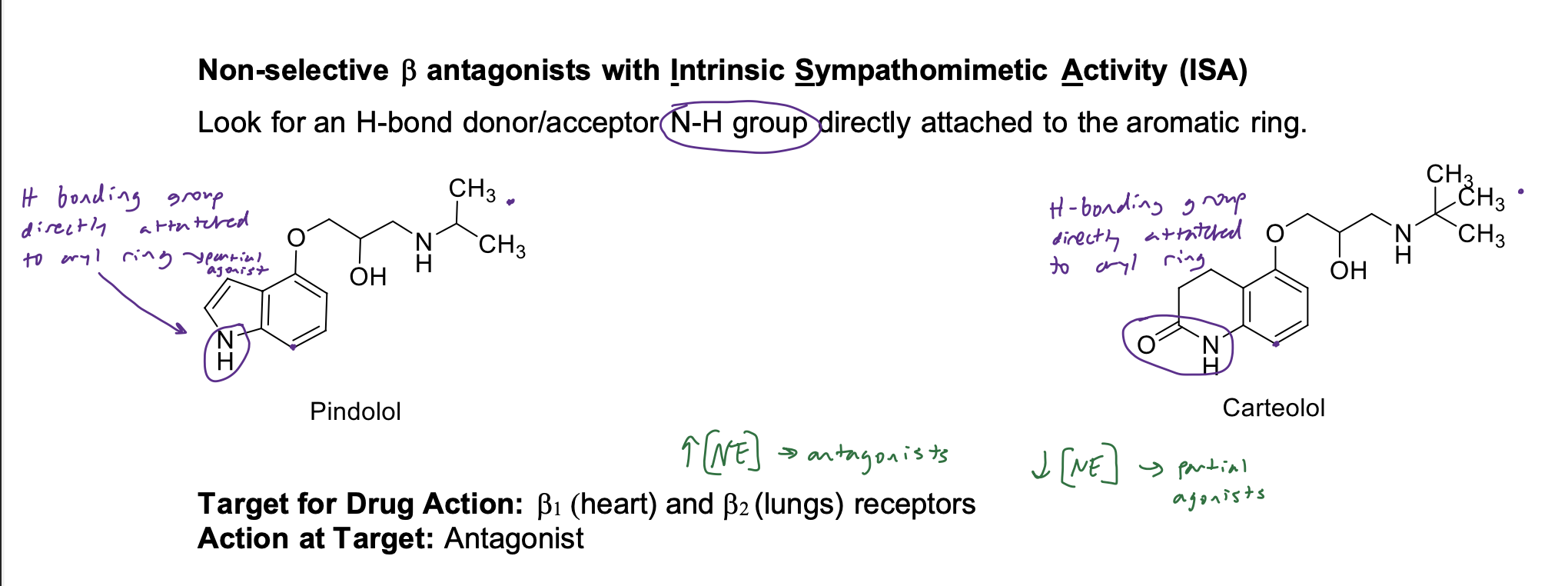
If there is less ISA, then what kind of agonist do you expect these drugs to be?
partial agonists
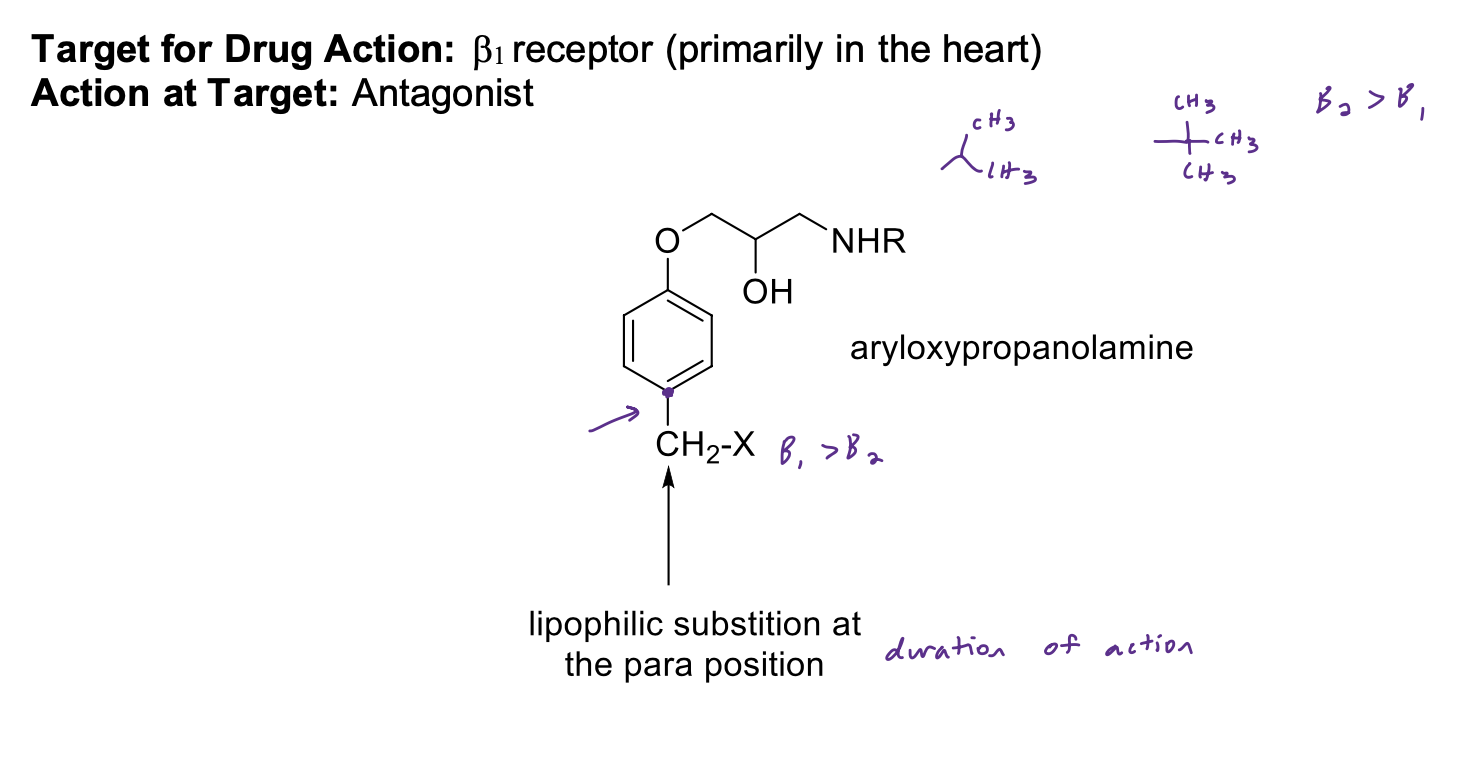
B1-Selective Antagonists (Cardioselective) SAR
• Aryloxypropanolamine; with lipophilic substituent para to the oxygen on the aryl ring. The nature of this para substituent also dictates the relative duration of the drug.
• Large branched alkyl group on the basic nitrogen (e.g., isopropyl)
• Basic nitrogen is required for binding; ionized form binds to receptor
• NO H-bond donor/acceptors directly attached to the aryl ring (except acebutolol) - (1 less point of interaction between drug + target, do not get activation)
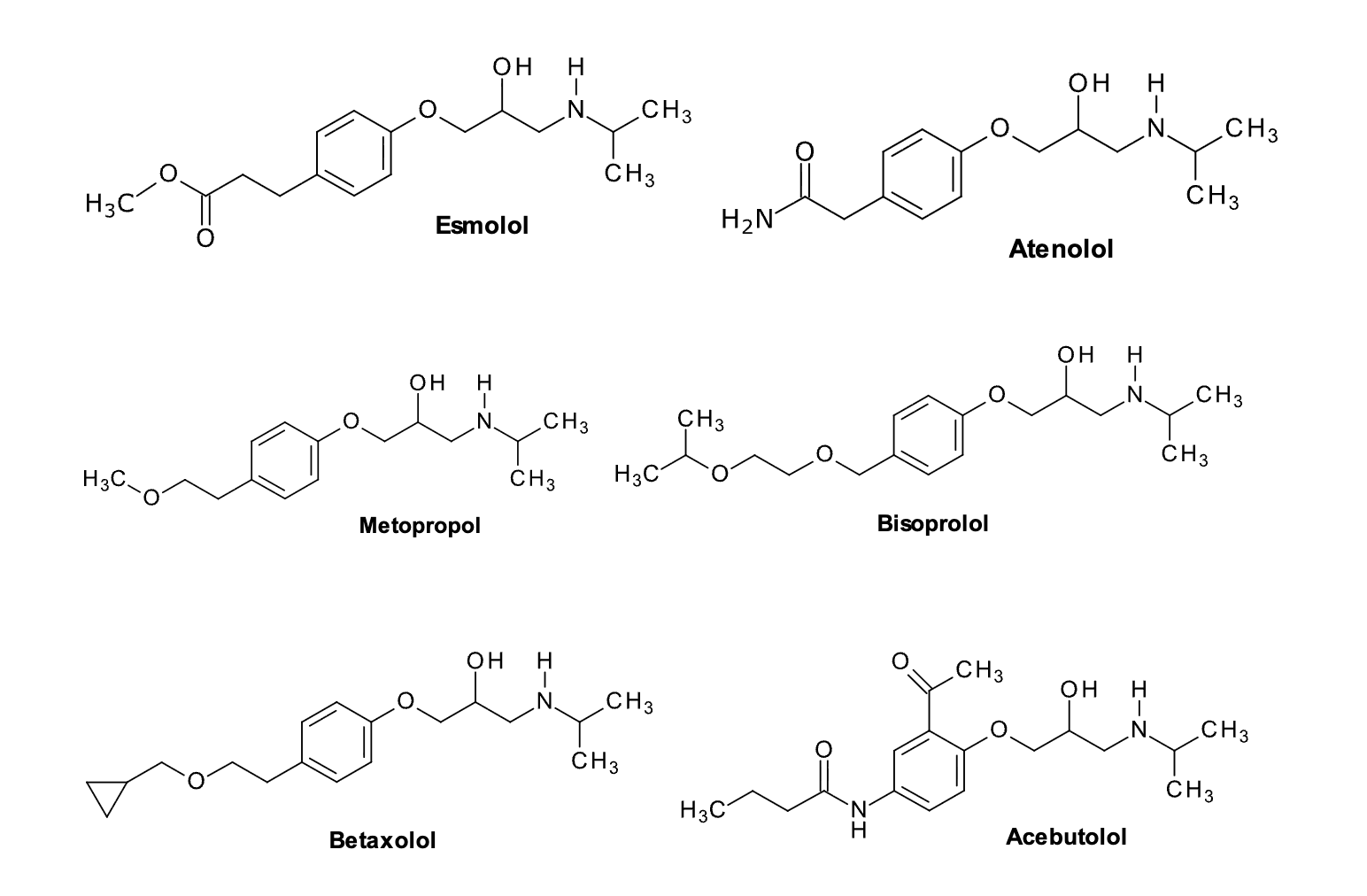
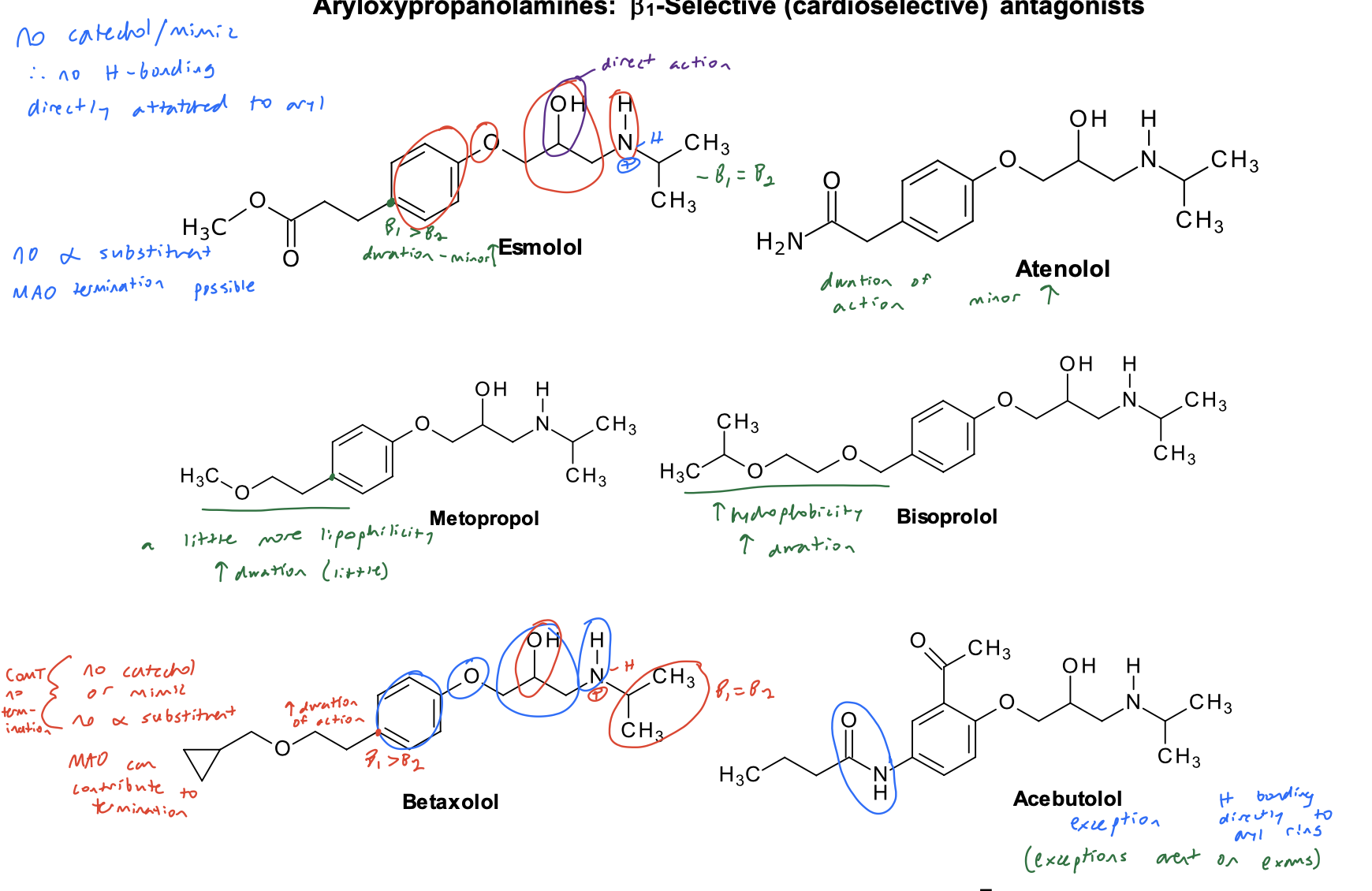
Aryloxypropanolamines: B1-Selective (cardioselective) antagonists
Combination a and b Antagonists Labetalol and Carvedilol
Target for Drug Action: a and b receptors
Action at Target: Antagonist
Alpha antagonism: decreases peripheral resistance
Beta antagonism: decreases rate and force of heart contraction
Combined effect is an effective control of BP

Important Structural Features - Labetalol
Phenethanolamine N-aralkyl group gives a1-
antagonism