CHEM 333 Acidity and Basicity (pKa) Table
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
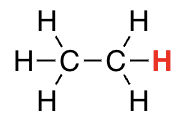
H on SP3 carbon
> 50
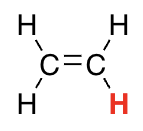
H on SP2 carbon
44
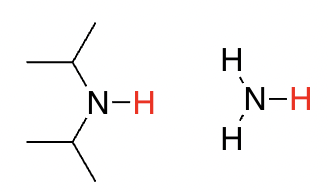
amines
35
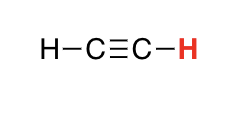
H on SP carbon
25
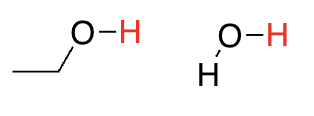
alcohols
16-18 (oxygen is more EN than carbon so hydrogens on oxygen are more acidic)
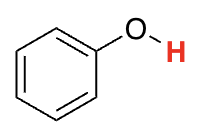
Phenols
9-10 (resonance)
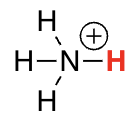
protonated amines
9
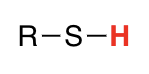
thiols
7
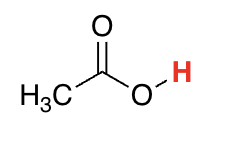
carboxylic acids
4-5 (because of the adjacent EWG [C=O] and resonance stabilization)
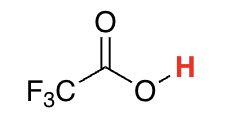
carboxylic acids with adjacent electronegative groups
0
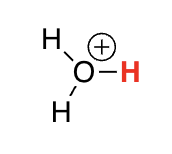
protonated alcohols
-1.7
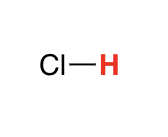
H-X
-7
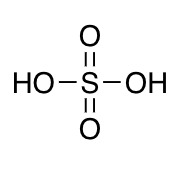
sulfonic acids
-9

Hydrogens on carbons adjacent to C=O
20-25 (C=O are EWGs and the anion that forms is stabilized by resonance)
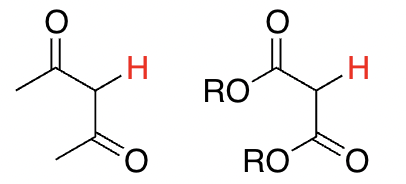
Hydrogens on carbons adjacent to two C=Os
9-10
the most important parameter in determining the acidity of a proton
the electronegativity of the atom directly connected to the proton
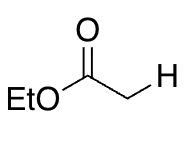
ester
25
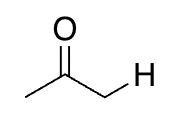
aldehyde/ketone
20
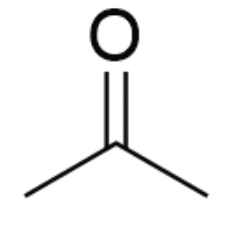
Name this compound.
acetone
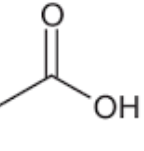
Name this compound.
acetate