AQA A level Chemistry 3.3.4 Alkenes
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Why are alkenes more reactive than alkanes? (2)
- Alkenes are more reactive because of the C=C double bond
- Which has a high electron density
What is an electrophile in the context of alkene reactions? (2)
- Electron-deficient species that accepts a pair of electrons
- Contains an atom with a partial positive charge (delta+)
What happens to the double bond (C=C) during an electrophilic addition reaction? (3)
1. The double bond breaks
2. The electrons are donated to the electrophile
3. Forming a single bond and saturated (C-C) products
How does the high electron density of the C=C bond contribute to the reactivity of alkenes? (1)
Makes the C=C bond readily attacked by electrophiles
What is the general equation for electrophilic addition reactions in alkenes? (1)
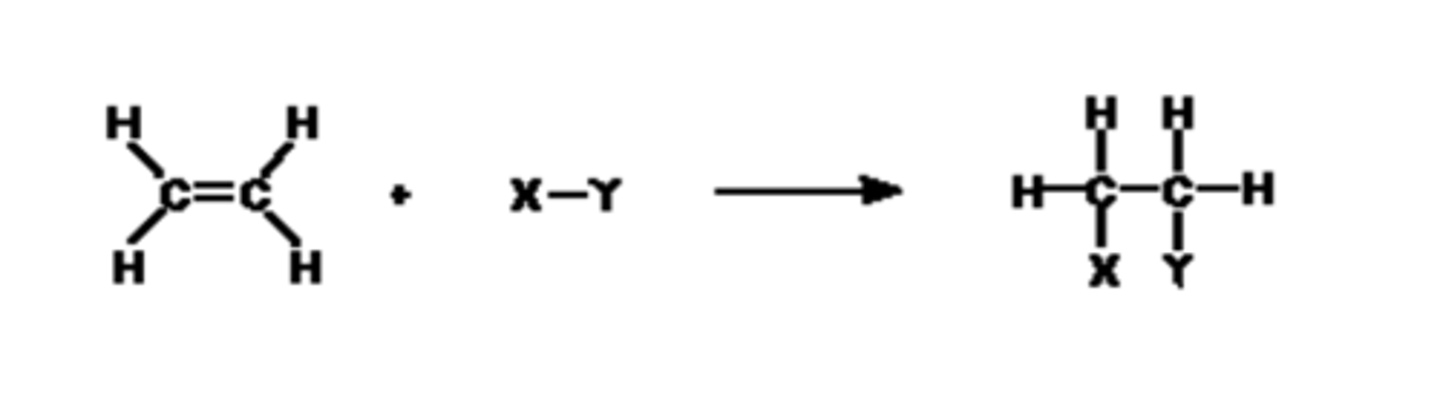
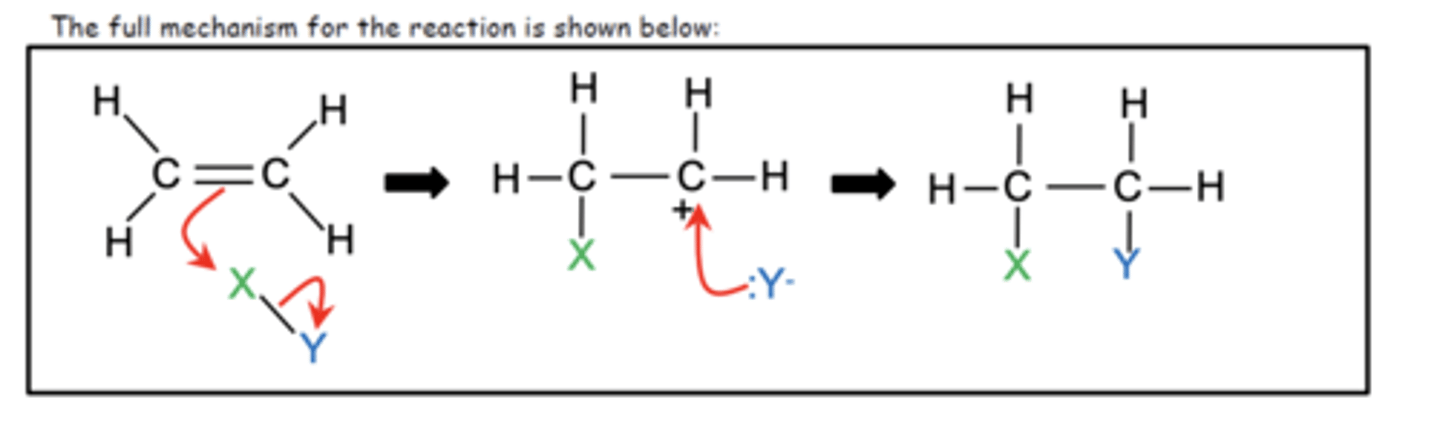
Draw the general mechanism for electrophilic addition reactions in alkenes? (3)
What is the test for alkenes using bromine water? (2)
- Add bromine water to the alkene.
- The red-brown colour will decolourise to show a carbon-carbon double bond
Write the equation for the reaction of ethene with bromine to form 1,2-dibromoethane. (1)
C2H4 + Br2 → C2H4Br2
Draw the mechanism for the electrophilic addition of bromine to ethene. (4)
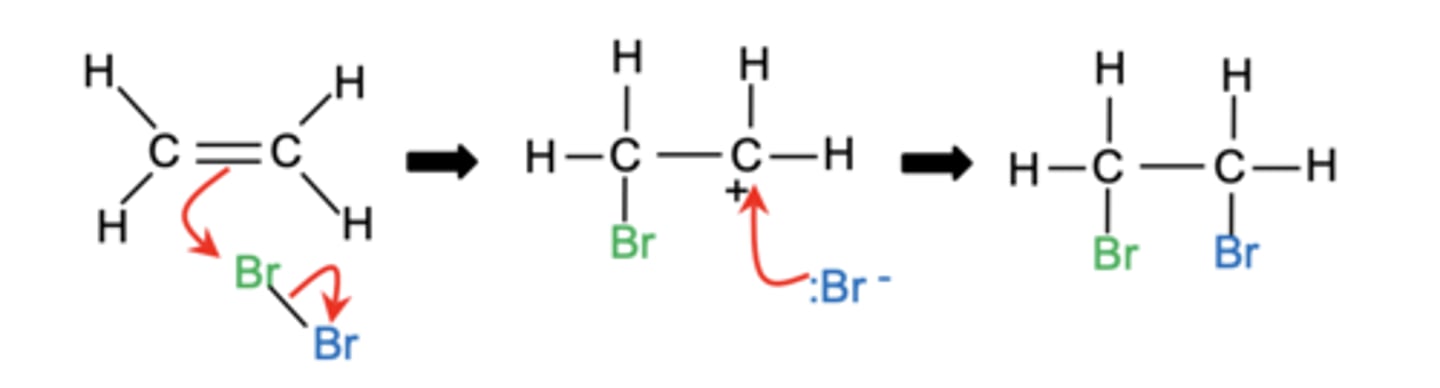
Describe the stages of the electrophilic addition mechanism of bromine to ethene. (4)
1. The electron-rich area of the double bond induces a dipole on the bromine molecule.
2. Electrons from the C=C move to δ+ Br, forming a carbon-bromine bond and releasing Br⁻.
3. The other carbon in the double bond becomes a carbocation.
4. The Br⁻ ion acts as a nucleophile, attacking the carbocation to form the final product
Draw the mechanism for the electrophilic addition of hydrogen bromide to ethene. (4)
Draw the mechanism for the electrophilic addition of sulphuric acid to ethene. (4)
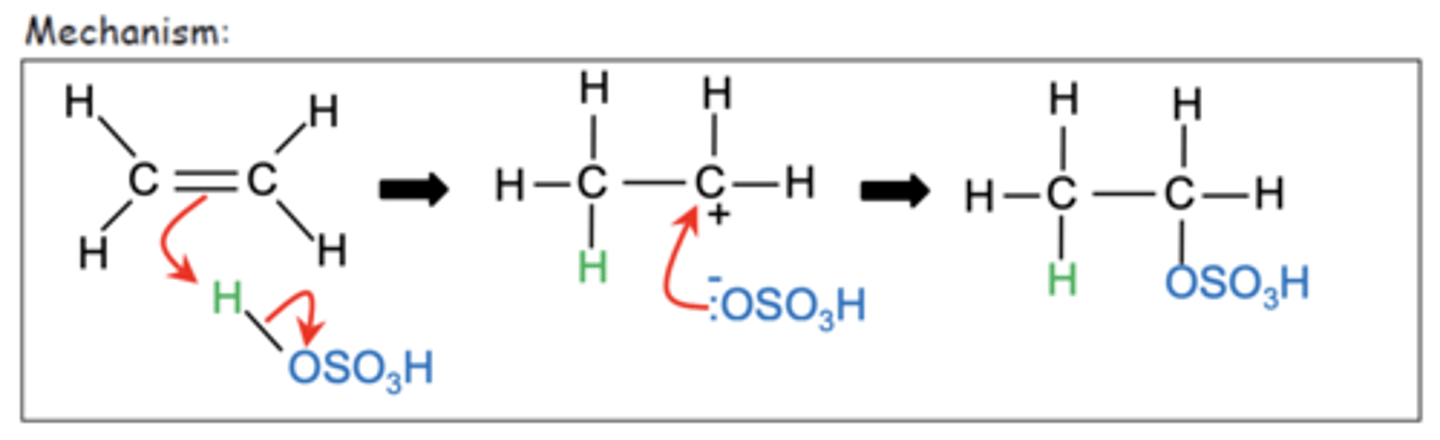
What is the product of hydrolysis of ethyl hydrogensulfate with water? (1)
Ethanol (CH3CH2OH)
Write the equation for the hydrolysis of ethyl hydrogensulfate with water. (1)
CH3CH2OSO3H + H2O → CH3CH2OH + H2SO4
What is the overall equation for the reaction of an alkene with concentrated H2SO4 followed by hydrolysis? (1)
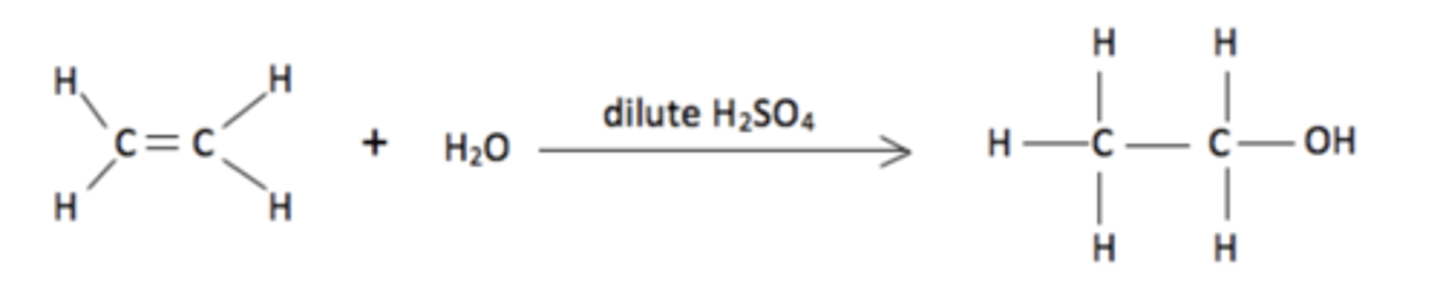
Why does sulfuric acid act as a catalyst in the overall reaction? (1)
Sulfuric acid is not used up in the reaction
What happens when alkenes react with electrophiles in an unsymmetrical environment? (1)
They form two products: a major product and a minor product
How is the major product in the electrophilic addition of unsymmetrical alkenes determined? (1)
From the more stable carbocation intermediate
What are the three types of carbocations in increasing order of stability? (3)
Primary (1°) < Secondary (2°) < Tertiary (3°)
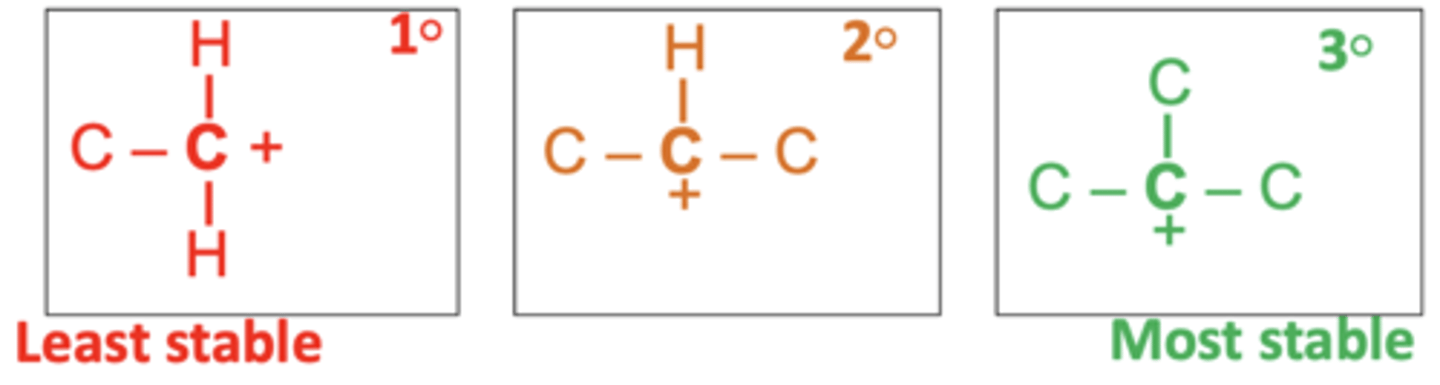
What makes tertiary carbocations more stable than primary carbocations? (2)
- Presence of more alkyl groups stabilizes the carbocation.
- Positive inductive effect of alkyl groups helps stabilise the positive charge
What is the positive inductive effect in carbocations? (1)
It is the electron-releasing property of alkyl groups that stabilises the positive charge on the carbocation
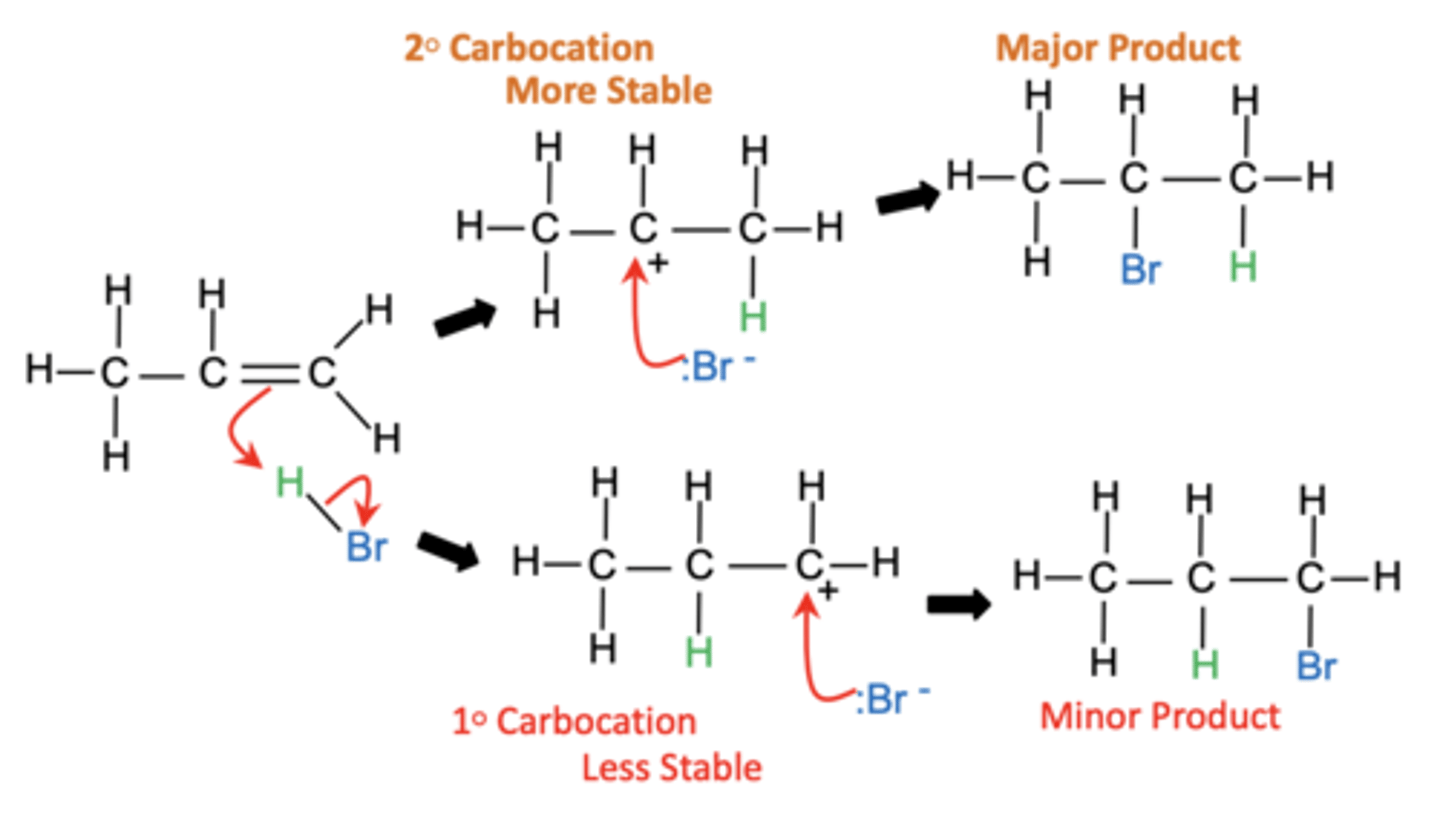
Why do unsymmetrical alkenes give rise to different products during electrophilic addition? (2)
- Different carbocation intermediates form during the reaction
- Leading to products in unequal amounts
Which product forms in higher amounts during electrophilic addition to unsymmetrical alkenes? (1)
The product derived from the more stable carbocation forms in higher amounts
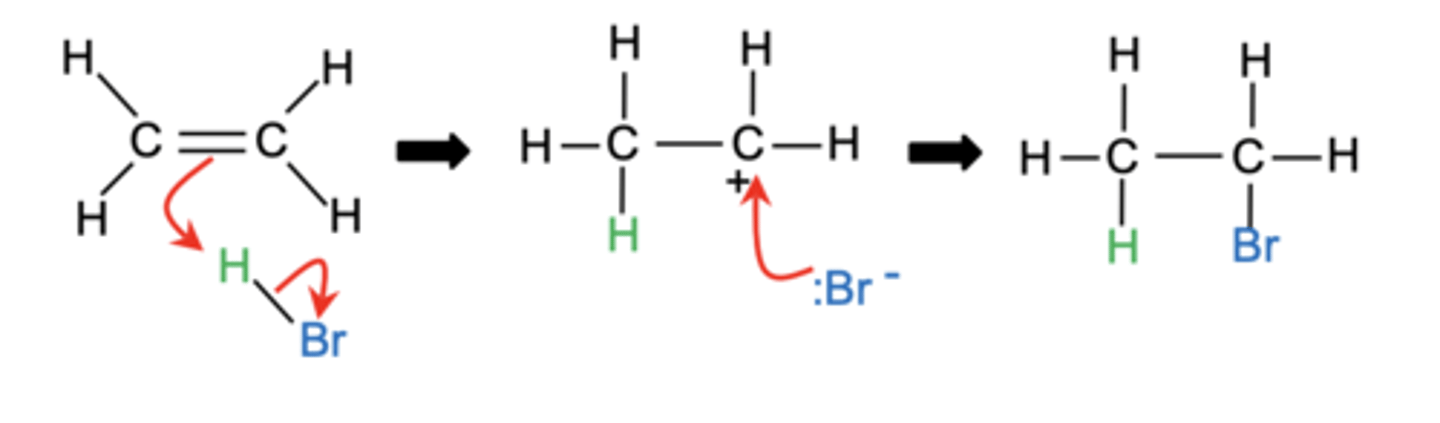
Draw the mechanism for the electrophilic addition of HBr to an unsymmetrical alkene, showing the formation of both major and minor products. (6)
What is addition polymerisation? (3)
- A reaction where small molecules (monomers, alkenes) join together
- To form a very large molecule called a polymer (poly(alkene))
- Which is unreactive
What is the polymer formed when ethene molecules join together? (2)
- The polymer formed is poly(ethene)
- Which is saturated (C-C bonds)
Draw the structure of the repeating unit of poly(ethene). (3)
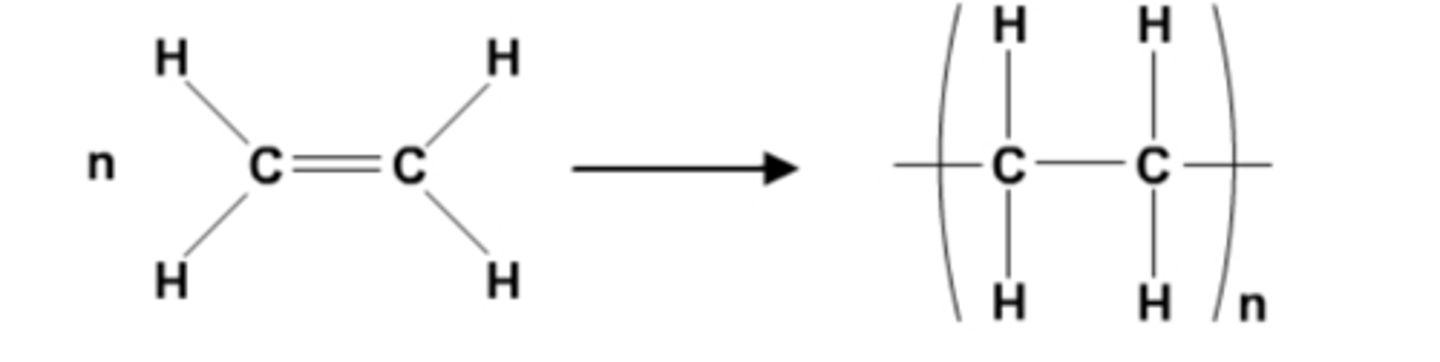
What is poly(chloroethene) commonly known as? (1)
PVC
Why does pure poly(chloroethene) tend to be hard and rigid? (1)
It has permanent dipole forces between chains due to the δ⁻ Cl and δ⁺ C
What is the purpose of plasticisers in PVC? (2)
- Added to reduce the effectiveness of intermolecular forces
- Making the plastic more flexible
What are the most common plasticisers used for PVC? (1)
Phthalates (esters of phthalic acid)
List one use of unplasticised PVC. (3)
Doors, windows, or guttering
List one use of plasticised PVC. (3)
Cable insulation, imitation leather, or inflatable products
Why are most plastics not biodegradable? (1)
They are not broken down by microbes quickly or at all
How can poly(ethene) be recycled? (1)
It can be separated, washed, melted, and remoulded into new products
How can poly(propene) be recycled? (1)
It can be heated to break polymer bonds, producing monomers that can be used to make new plastics.