Chemistry 3.5- Alcohols
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
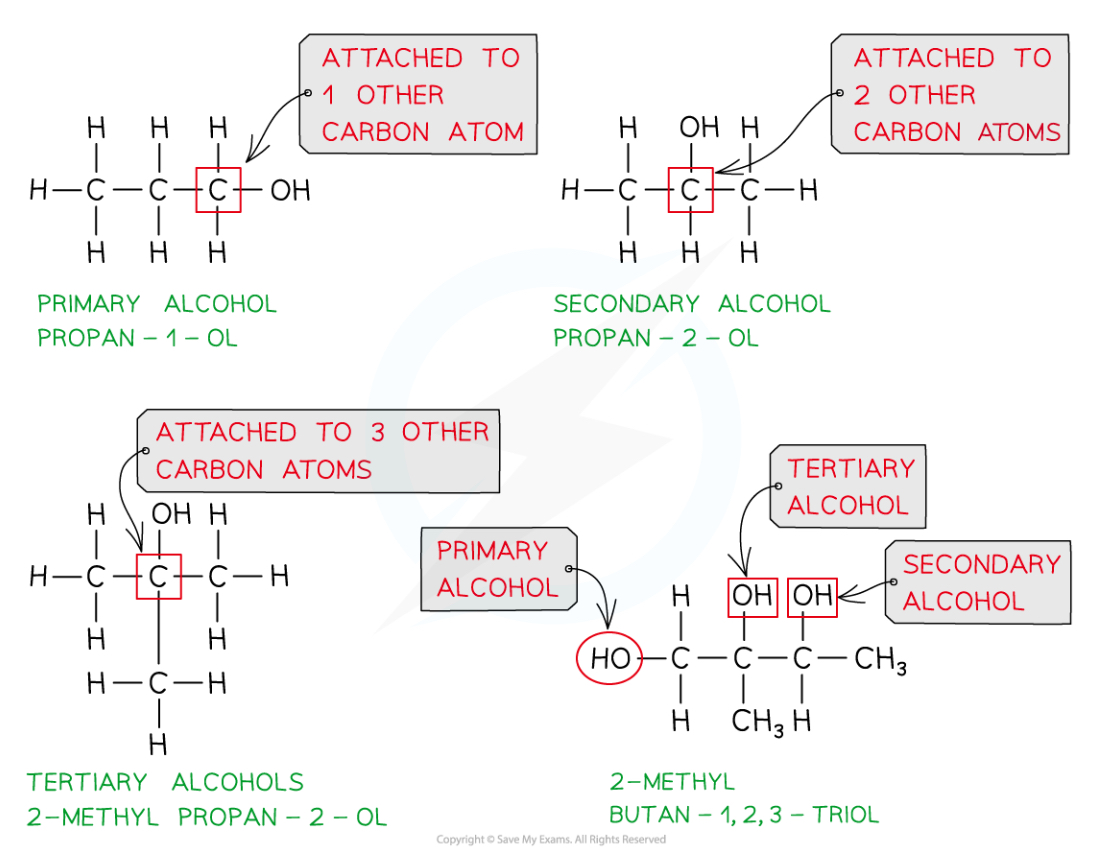
How can we classify alcohols?
Primary alcohols- the carbon atom bonded to the -OH group is attached to one other carbon atom
Secondary alcohols- the carbon atom bonded to the -OH group is attached to two other carbon atoms
Tertiary alcohols- the carbon atom bonded to the -OH group is attached to three other carbon atoms
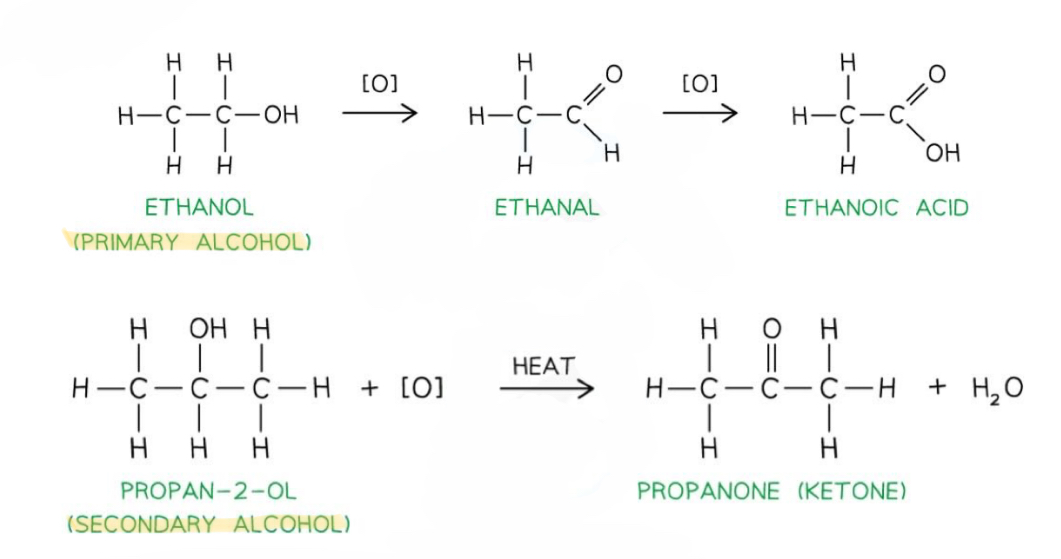
Which alcohols can be oxidised and what to?
Primary alcohols can be oxidised into aldehydes, which can be further oxidised into carboxylic acids
Secondary alcohols can be oxidised into ketones
Tertiary alcohols can’t be oxidised
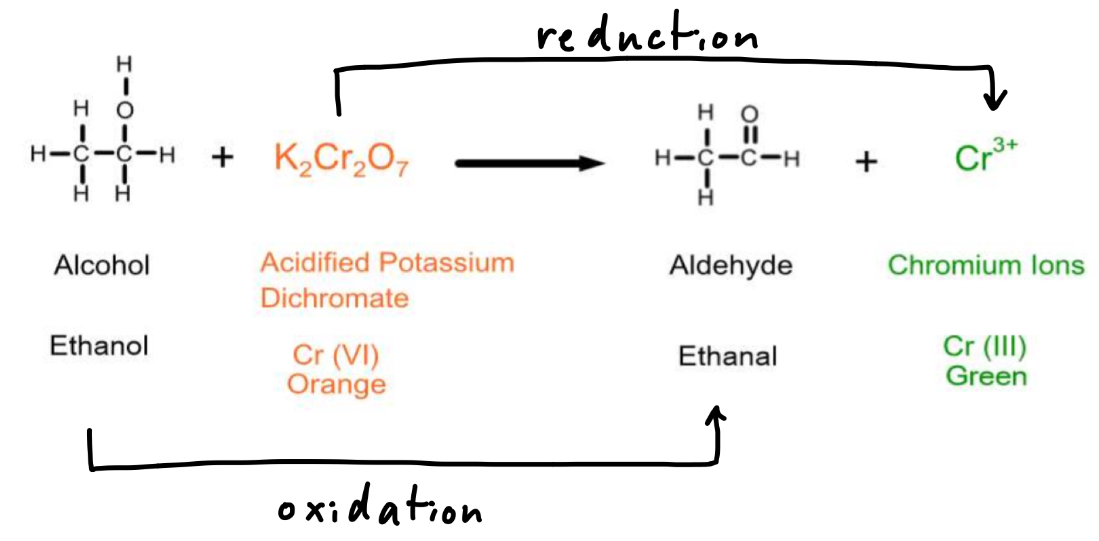
What can be used to oxidise alcohols and what change occurs?
Acidified potassium dichromate (VI)
The orange Cr 6+ ions are reduced to green Cr 3+ ions when alcohols are oxidised
So the solution turns from orange to green
What colour flame do alcohols burn with?
Blue, but is often hard to see
What are the physical properties of alcohols?
The polar hydroxyl group can form hydrogen bonds, so they have higher melting and boiling points than alkanes with similar RFMs
However, the non-polar hydrocarbon chain can’t form hydrogen bonds, so shorter chain alcohols are soluble in water due to the hydroxyl group, while longer chains aren’t, as the non-polar end takes dominance
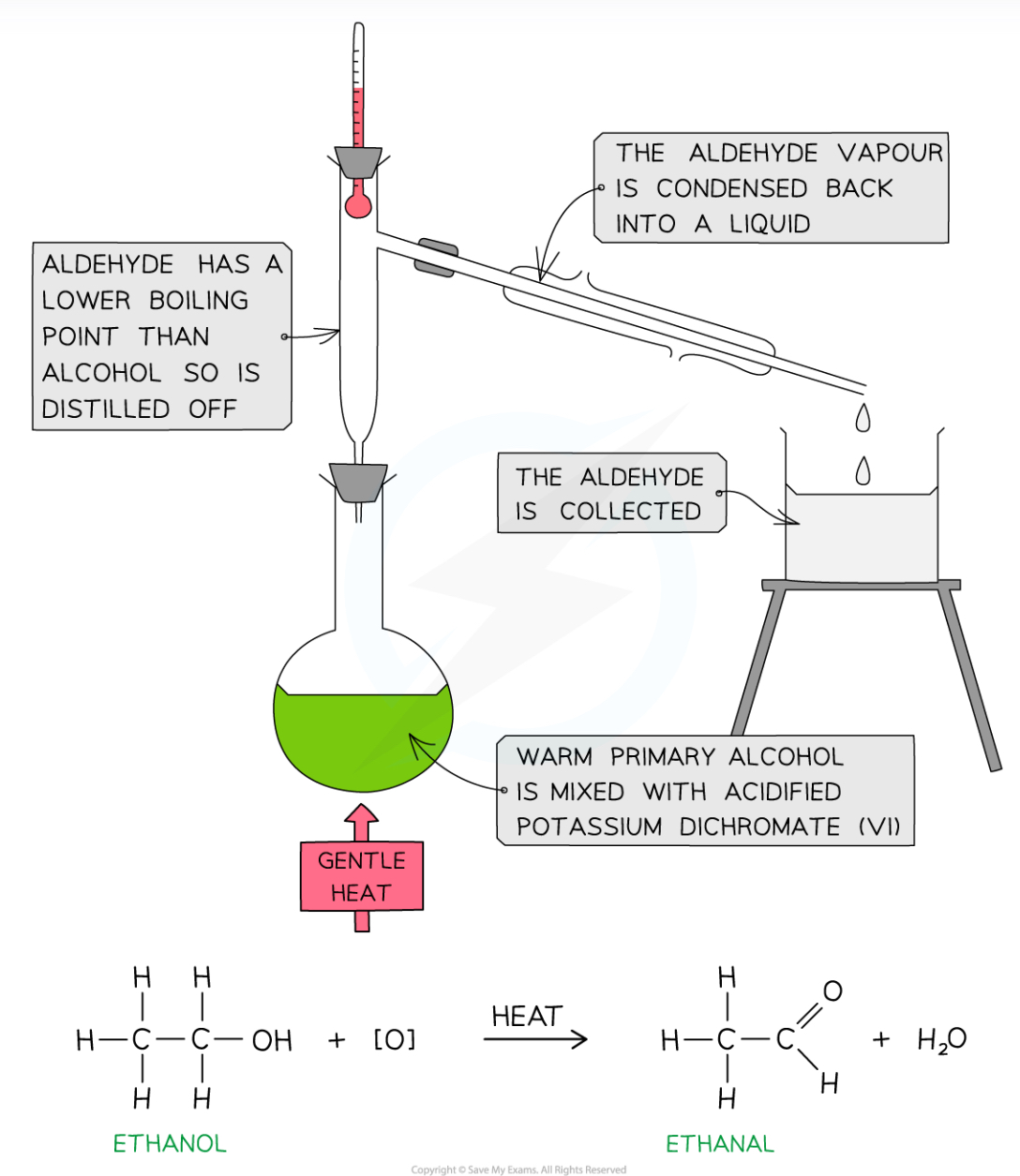
How can we produce aldehydes from alcohols? Why does this work?
Distillation- the alcohol can be heated gently with a small amount of potassium chromate (oxidising agent) and dilute sulfuric acid, and the aldehyde can be distilled out, without collecting a carboxylic acid
This is because aldehydes have no hydroxyl group, so they don’t form H bonds, so they have much lower boiling points than alcohols and carboxylic acids, and will evaporate first
To prevent the aldehyde evaporating off, the receiver is cooled in an ice bath
How can we produce carboxylic acids from alcohols? Why does this work?
Reflux- the alcohol can be heated with an excess of potassium chromate (oxidising agent) and dilute sulfuric acid, and allowed to oxidise until the mixture is fully carboxylic acid
This works because the aldehyde produced evaporates, cools in the condenser and falls back into the flask to be further oxidised to a carboxylic acid
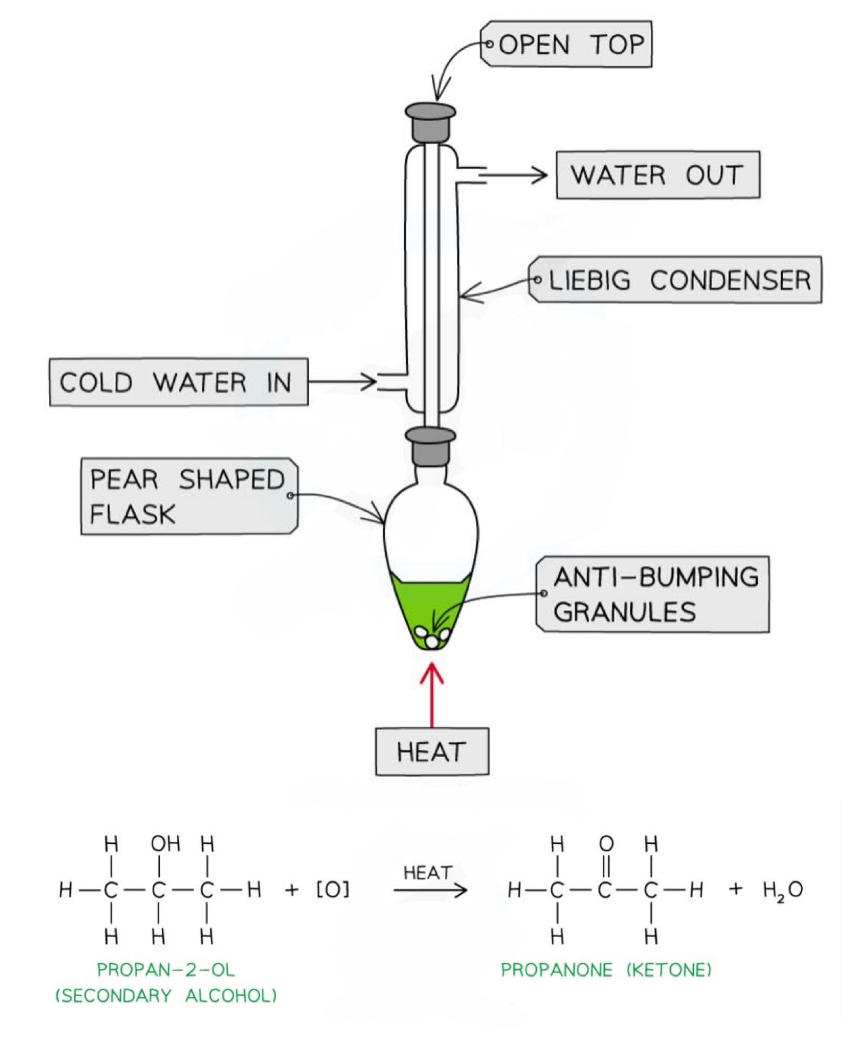
How can we test to distinguish between aldehydes and ketones?
Fehling’s solution- turns from clear blue to a red precipitate when heated gently with an aldehyde (as the blue Cu 2+ ions oxidise the aldehyde and are themselves reduced to Cu + in the red precipitate Cu2O)
Tollen’s solution- turns from clear and colourless to a silver mirror when heated gently with an aldehyde (as the dissolved Ag + ions oxidise the aldehyde and are themselves reduced to insoluble Ag atoms)

How can we test for carboxylic acids?
Carboxylic acids will neutralise sodium carbonate solution to produce bubbles of carbon dioxide
What is the general oxidation equation for alcohols into aldehydes?
RCH2OH + [O] → RCHO + H2O
What is the general oxidation equation for alcohols into carboxylic acids?
RCH2OH + 2[O] → RCOOH + H2O
What is the general oxidation equation for aldehydes into carboxylic acids?
RCHO + [O] → RCOOH
What is the general oxidation equation for alcohols into ketones?
RCHOHR + [O] → RCOR + H2O
Outline the mechanism for the dehydration of alcohols into alkenes by elimination
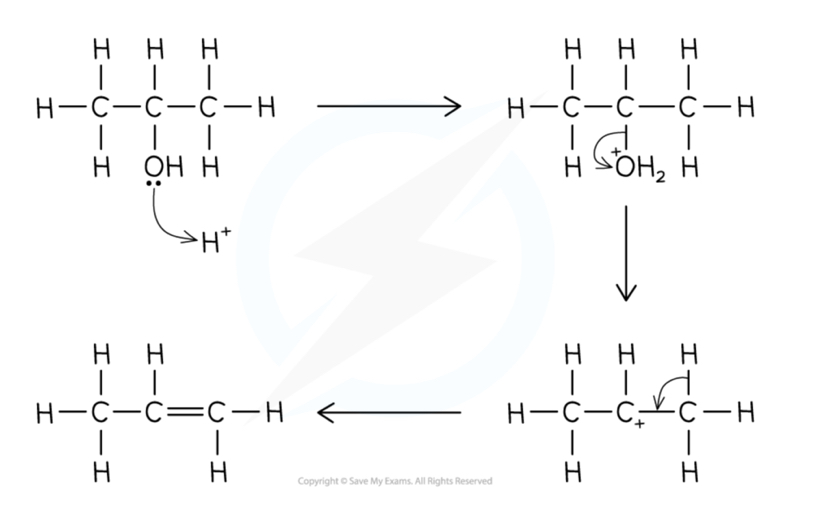
What are the conditions for the dehydration of alcohols into alkenes?
Heating the alcohol and passing the vapours over a hot catalyst such as concentrated phosphoric acid or aluminium oxide
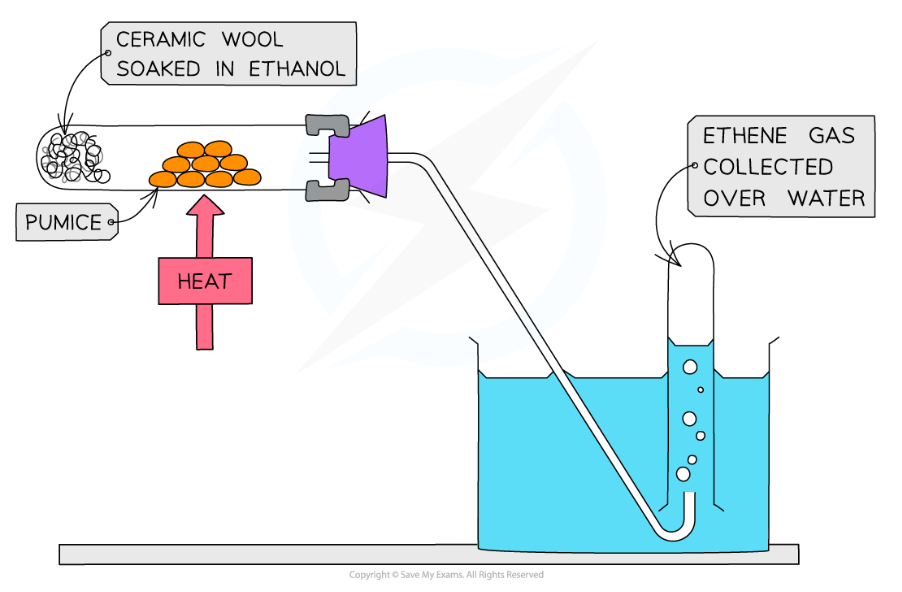
Why is the dehydration of alcohols to alkenes useful?
It produces alkenes that can be used to form addition polymers, without using monomers derived from crude oil
How can we produce ethanol by fermentation? Include the formula
Fermenting glucose (usually from sugarcane) with yeast
35°C
Anaerobic conditions (prevents oxidation to carboxylic acid)
At around 15% ethanol the yeast dies, so fractional distillation is used to produce higher concentrations
C₆H₁₂O₆ —> 2C₂H₅OH + 2CO₂
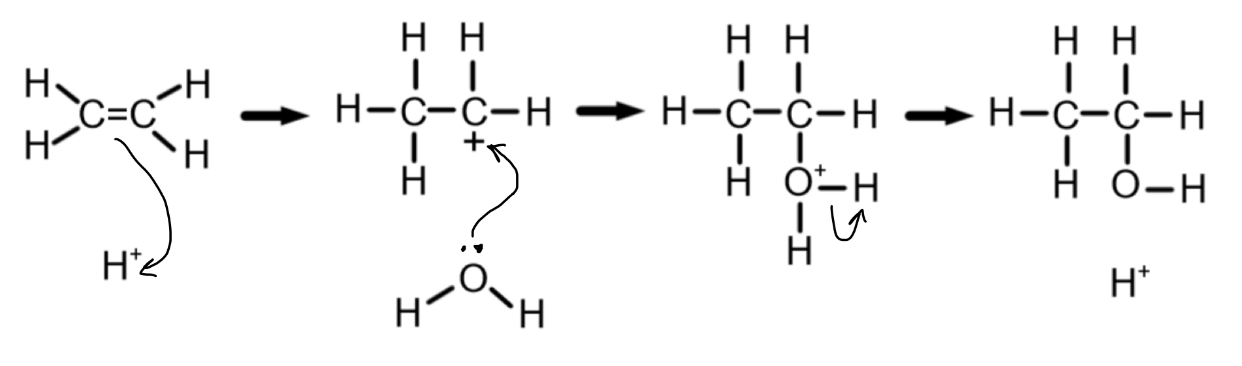
How can we produce ethanol from crude oil?
Long hydrocarbon chains can be cracked to produce ethene
Ethene can undergo hydration (addition of water) to form ethanol, in the conditions:
300-600
50-100 atm
Concentrated phosphoric acid catalyst
Steam
What are the positives and negatives of producing ethanol by fermentation?
Pros
It is renewable as sugarcane is very quick to grow- crude oil is not renewable as it takes millions of years to form
It can be viewed as carbon neutral as the carbon dioxide released when ethanol is combusted is balanced by the carbon dioxide absorbed through photosynthesis of the sugarcane
Cons
In practice it isn’t fully carbon neutral, considering transportation, irrigation and processing (fractional distillation)
The monoculture production of sugarcane takes up lots of deforested land in rainforests and reduces biodiversity
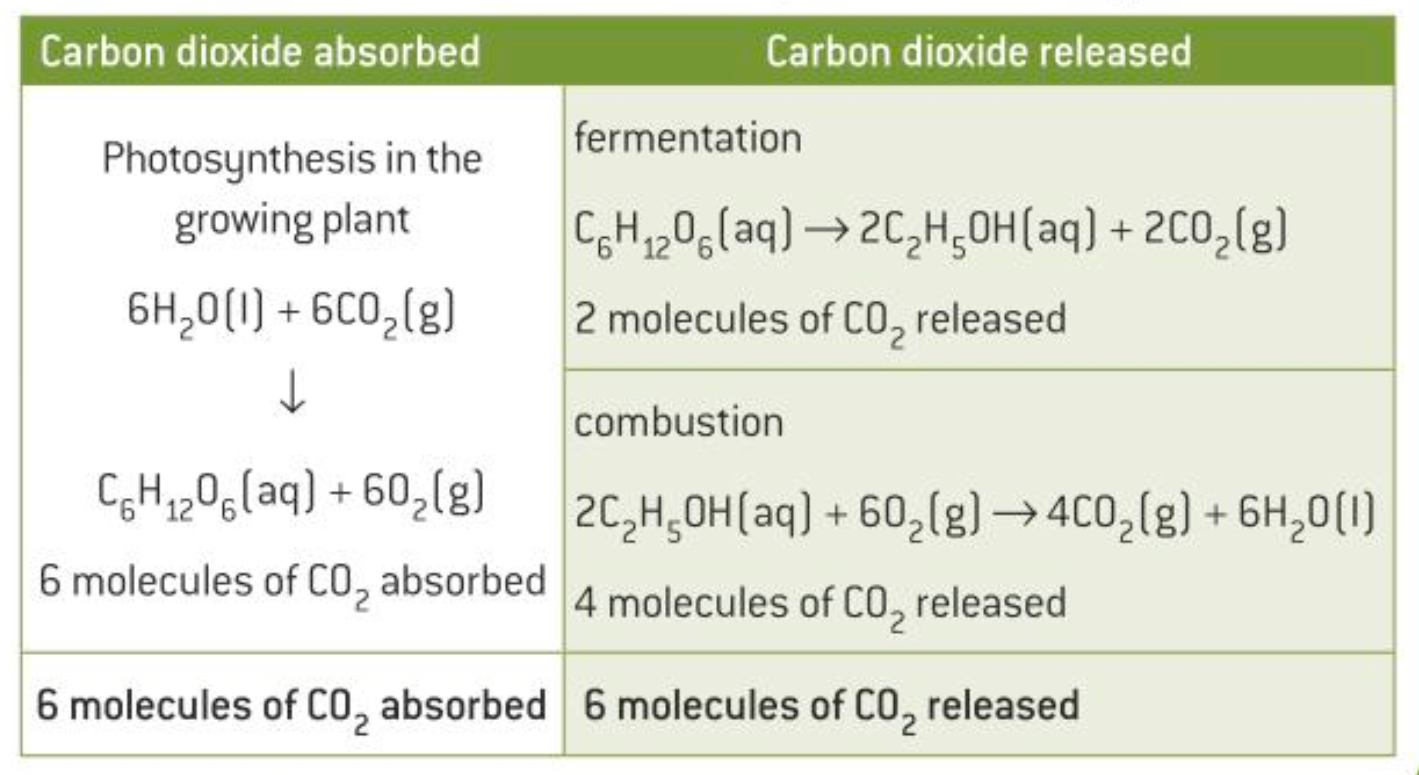
What are biofuels?
Fuels produced from biomass eg. plants or algae