AP Chemistry Final
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
The volume of a gas is proportional to the temperature of the gas is known as..
Charles’ Law
The volume of a gas is inversely proportional to the pressure of a gas is known as
Boyle’s Law
Near Cozumel, Mexico, a scuba diver goes down 60 ft to look at the sea creatures. As she defends, the air pressure increases. Which gas law is this an example of?
Boyle’s Law
A large balloon is initially filled to a volume of 25.0L at 353K and a pressure of 2575mm Hg. What volume of gas will the balloon contain at 1.35atm and 253K
45.0L
A syringe initially holds a sample of gas with a volume of 285mL at 355K and 1.88atm. To what temperature must the gas in the syringe be heated/cooled to in order to have a volume of 435mL at 2.50atm?
721 K
What pressure will 14g of CO exert in a 3.5L container at 75C
4.1 atm
What mass of NO2 is contained in a 13.0 L tank at 4.58 atm and 385 K
86.7 g

A
A .334 g sample of an unknown halogen occupies 109mL at 398 K and 1.41 atm. What is the identity of the halogen?
Cl2
A compound is found to be 30.45% N and 69.55% O by mass. If 1.63 g of this compound occupy 389mL at 0.00C and 775mm Hg, what is the molecular formula of the compound?
N2O2
To avoid oxygen toxicity and nitrogen narcosis, deep-sea divers breathe a specialized mixture of gas. Describe
Helium and oxygen
A syringe contains 589mL of CO at 325K and 1.2atm pressure. A second syringe contains 473mL of N2 at 298 K and 2.6atm. What is the final pressure if the contents of these two syringes are injected into a 1 L container at 0.00C
1.7 atm
Determine the volume of O2 (at STP) formed when 5.0g of KClO3 decomposed according to the following reaction
13.7 L

32.9 L
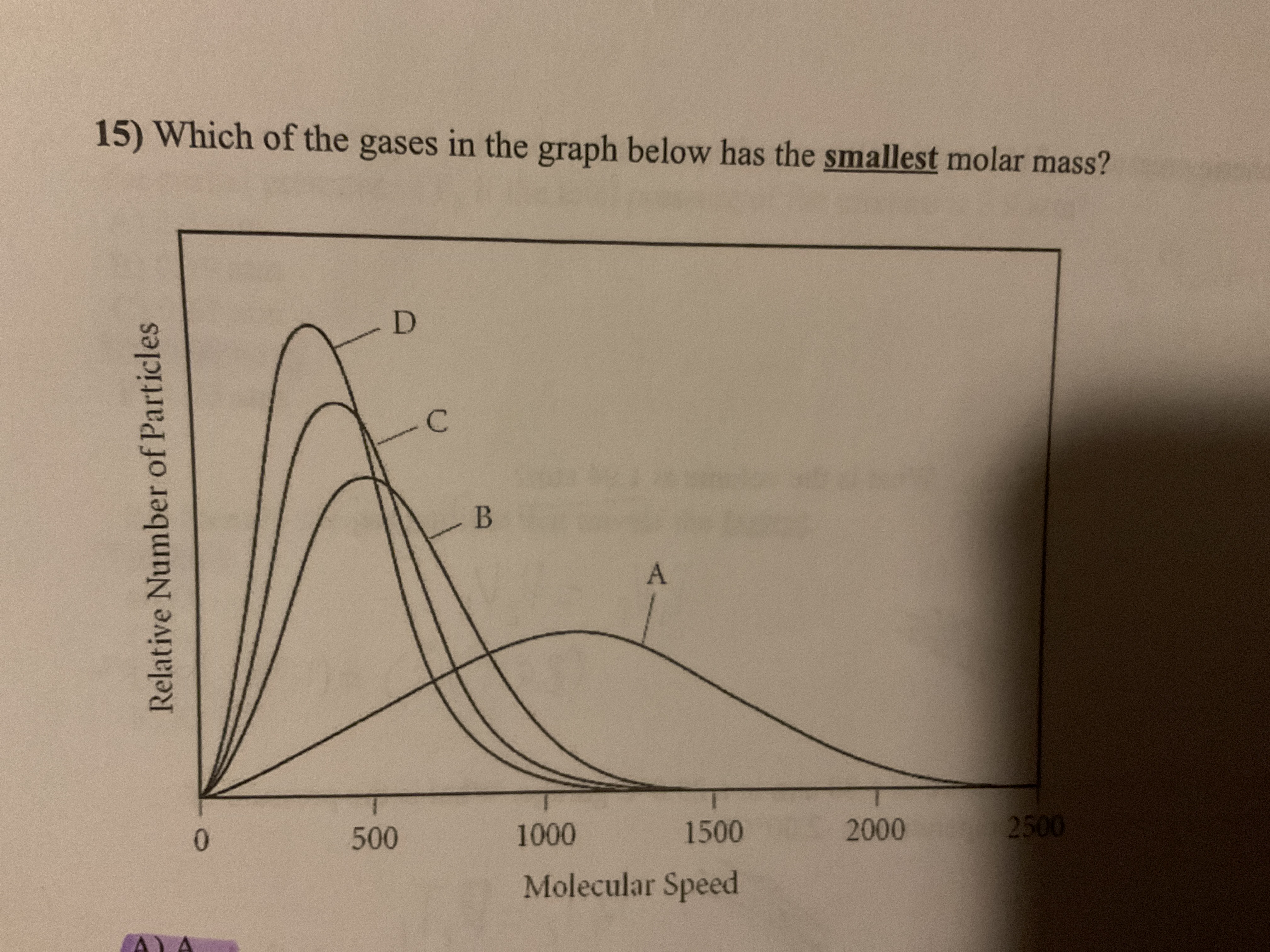
A
Calculate the root mean square velocity of nitrogen molecules at 25C
515 m/s
Convert 2 atm to torr
1520 torr
The atmospheric pressure is 715 mm Hg. What is the pressure in torr
715 torr
A gas occupies 2.22 L at 3.67 atm. What is the volume at 1.94 atm
4.21 L
A basketball is inflated to a pressure of 1.80 Amy in a 23 C garage. What is the pressure of the basketball outside where the temp is -2.00C
1.65 atm
What volume will .875 moles of Kr occupy at STP
19.6 L
The density of Krypton gas at 1.35 atm and 54.1 C is
4.21g/L
A mixture of F2, H2, Xe have mole fraction of .25, .65, and .10 respectively. What is the partial pressure of F2 if the total pressure is 3.9 atm
.98atm
Identify the gas particle that travels the fastest
H2
If a 600.