Cluster 5: Tumour Immunology (Dr. Nisha)
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
What is the definition of tumours
Tumours are abnormal masses of tissue that arise from uncontrolled cell division, which can be benign or malignant.
They can invade surrounding tissues and potentially spread to other parts of the body (metastasis).
What is the meaning of clonal in relation to tumours
Clonal in relation to tumours refers to the concept that all cells in a tumour originate from a single progenitor cell, leading to a uniform genetic makeup among the cancer cells.
What is the meaning of somatic in relation to tumours
Somatic in relation to tumours refers to mutations that occur in non-germline cells, which can contribute to the development of cancer without being inherited.
What are some of the factors that can cause somatic mutations
Ageing
Exposure to Chemicals
Radiation
Genetic
What type of cancers do EBV and HPV cause
HPV: High-risk HPV strains are the major causes of cervical cancer and other anogenital neoplasms as well as a significant proportion of head and neck tumours.
EBV: EBV is associated with nasopharyngeal carcinoma, Hodgkin’s lymphoma, and Burkitt’s lymphoma.
What are unique tumour antigens
Unique tumour antigens are proteins expressed on the surface of cancer cells that are not found on normal cells, making them potential targets for immunotherapy.
Proteins altered by mutation
– Viral antigens
– Fusion proteins from chromosomal aberrations
Explain the three types of unique tumour antigen
Possibility 1: Proteins Altered by Mutation
A mutation changes the structure of a normal protein.
This altered protein looks “foreign” to the immune system.
It can sometimes drive cancer (oncoproteins), or just be a marker of cancer.
Example: Mutated p53 or RAS
Possibility 2: Viral Antigens
Some cancers are caused by viruses (e.g. HPV, EBV).
The virus makes the cell produce viral proteins — these are not human proteins.
Since they are foreign, they’re good targets for the immune system.
Example: E6/E7 proteins from HPV in cervical cancer
Possibility 3: Fusion Proteins from Chromosomal Aberrations
Two different genes from different chromosomes fuse together abnormally.
This creates a new “fusion” protein that doesn’t exist in healthy cells.
This new protein can be cancer-causing and is a unique marker.
Example: BCR-ABL fusion in Chronic Myeloid Leukaemia (CML) from t(9;22) — the Philadelphia chromosome
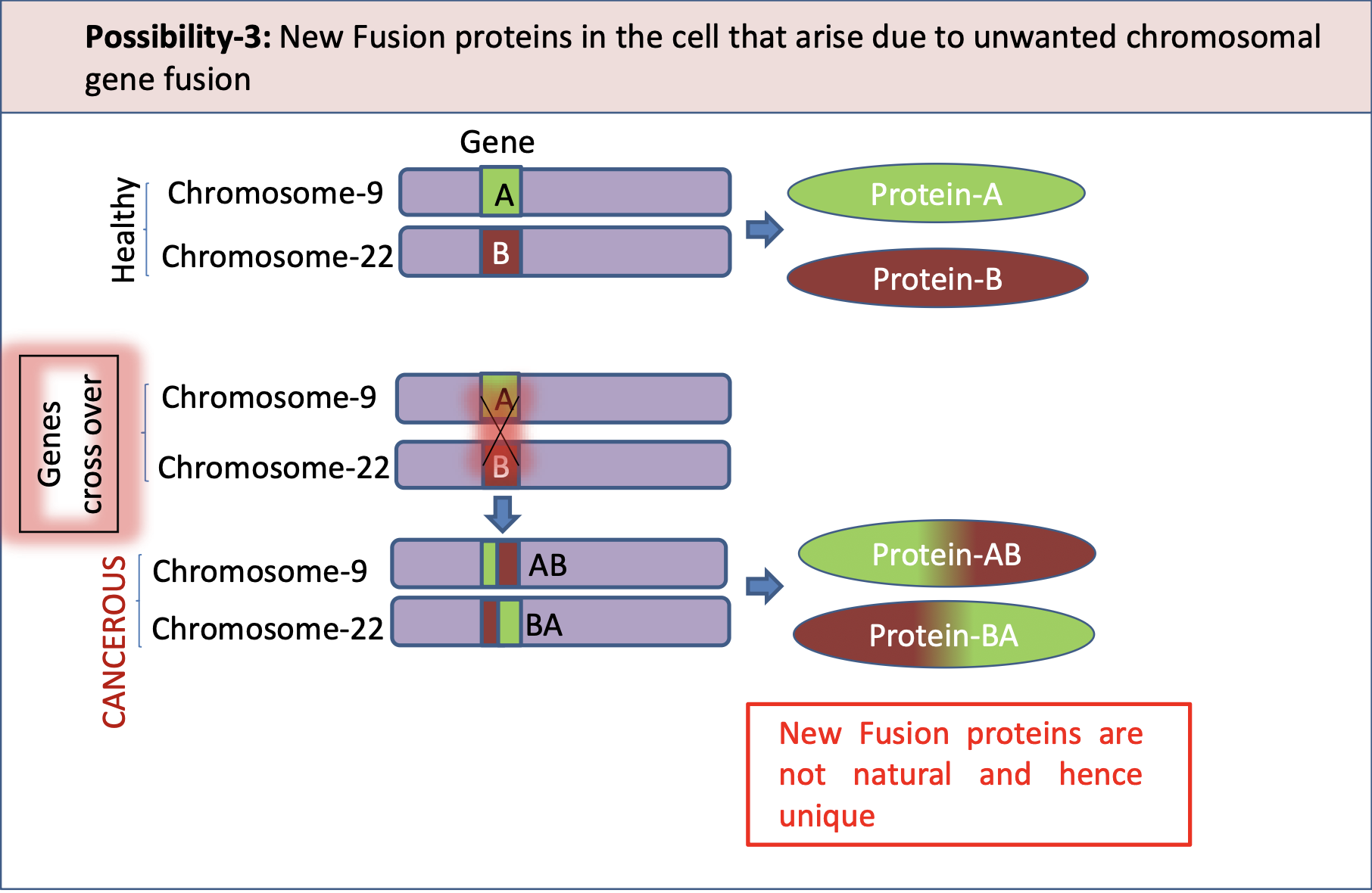
What is ABL1
ABL1 is a protein-tyrosine kinase involved in cell signalling and regulation.
It is a proto-oncogene, that could become an oncogene, due to mutations or increased expression
Often associated with chronic myeloid leukemia due to its role in fusion with the BCR gene.
What is BCR
BCR is a gene that codes for kinase, but its functions are unclear.
BCR is a gene that codes for a protein involved in signal transduction in immune cells, playing a crucial role in B-cell receptor signalling.
What are the function of the kinase enzymes
Kinase enzymes function to transfer phosphate groups from ATP to specific substrates, thus modifying and activating or deactivating proteins, which plays a critical role in regulating various cellular processes such as metabolism, cell division, and signal transduction.
What are the three unique tumour antigens
Evaluate the use of each tumour antigen as a diagnostic marker
What are differentiation antigens
These antigens are expressed in “tumours” and a “LIMITED RANGE OF NORMAL TISSUES” [or in other words] Expressed in “tumours” and in some normal human tissues, but not all human tissues
Differentiation antigens can be diagnostic markers; but if targeted in clinical trials → risk of inducing autoimmunity
e.g. melan-A
What is melan-A
melan-A (Melanoma-Antigen Recognized by T-cells 1 or MART-1) is normally expressed in cells of melanocyte lineage
e.g. in the skin, and retina but not in other normal tissues.
But if expressed in other cells (like mouth, intestine, legs), it is melanoma (cancer) and in those cancer cells, they are called as Differentiation antigens.
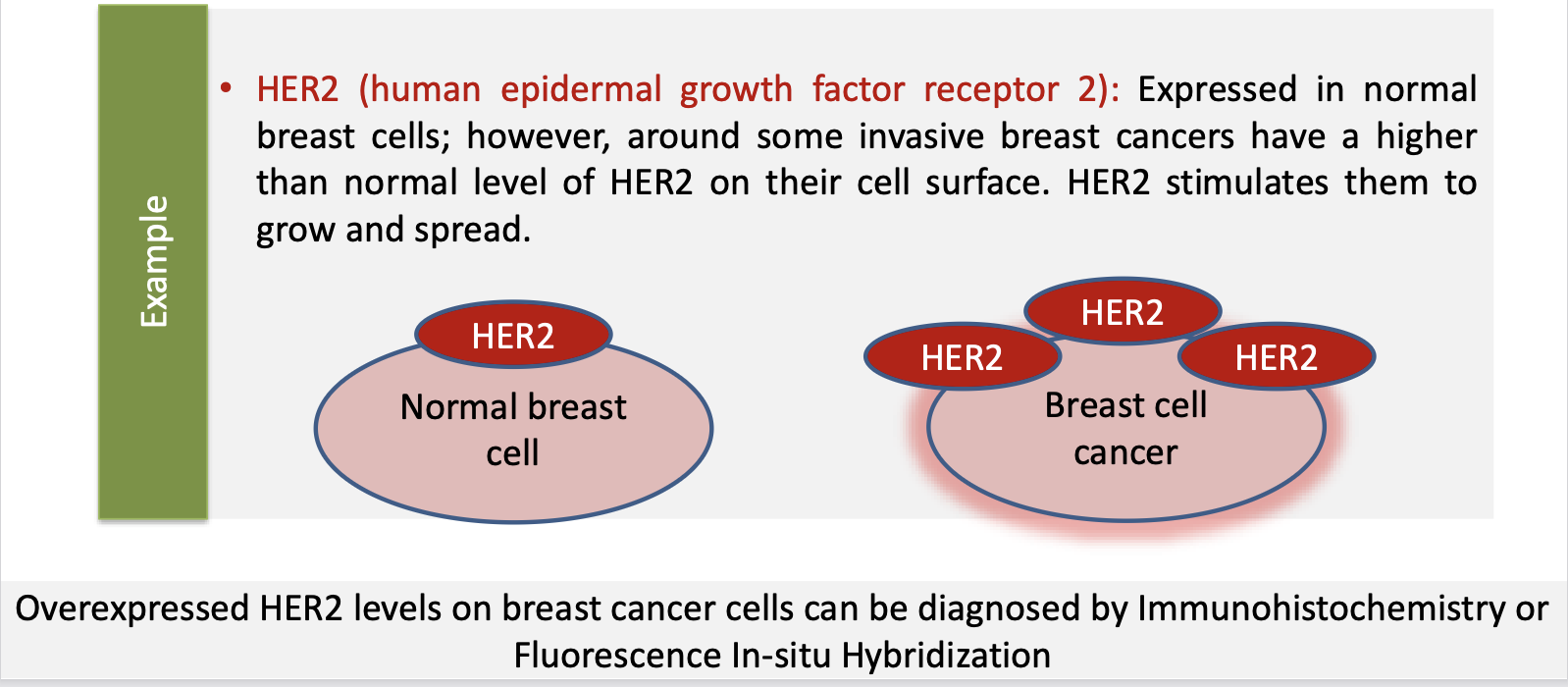
What are overexpressed antigens
Expressed by both normal and tumour cells, however, the difference is that these antigens are OVER EXPRESSED (expressed in higher levels) in tumour cells
– E.g. HER2, MUC1
What are HER2
Expressed in normal breast cells; however, around some invasive breast cancers have a higher than normal level of HER2 on their cell surface. HER2 stimulates them to grow and spread
Overexpressed HER2 levels on breast cancer cells can be diagnosed by Immunohistochemistry or Fluorescence In-situ Hybridization
What is the meaning of immune evasion
Immune evasion refers to the strategies used by tumour cells to avoid detection and destruction by the immune system, allowing them to survive and proliferate despite an immune response.
How are t-cells, b-cells activated
T-cells and B-cells are activated through antigen recognition, where T-cells require antigen presentation by major histocompatibility complex (MHC) molecules.
While B-cells can recognize free antigens. This activation process is further enhanced by co-stimulatory signals and cytokines from helper T-cells.
What are the two mechanisms of tumour evasion
Intrinsic to tumour cells
Loss of antigen
Hidden cell surface antigens
No expression of MHC I or other co-stimulators
Engage inhibitory molecules
Secrete factors that suppress anti-tumour immunity
Extrinsic: Mediated by other cells
T-regulatory cells suppress T cell response to tumour
Myeloid-derived suppressor cells (MDSCs) are immunosuppressive;
They interact with T-cells, Dendritic cells and Macrophages and Natural
Killer cells to regulate their function.
What is the difference between passive and active immunotherapy
Passive immunotherapy involves administering pre-formed antibodies to the patient, providing immediate but temporary protection.
In contrast, active immunotherapy aims to stimulate the patient's own immune system to produce a response against the tumour, typically through vaccines or other agents.
What are monoclonal antibodies (Mab)
Monoclonal antibodies provide targeted immunotherapy.
For example, directed to a single target on a cancer cell, usually an antigen or a receptor site on the cancer cell
What are the limitations of Mab
Mabs are not administered as first-line therapy
Cancer antigens may not be same in different patients and hence Mabs are effective only in 20-30% of cases
Tumour cells mutate as a result of radiation and
chemotherapy and Mab target is not availableMabs can be toxic (e.g. autoimmune diseases)
What are the advantages of Mab
Monoclonal antibodies (Mabs) offer targeted therapy for cancer, leading to fewer side effects compared to traditional chemotherapy.
They can enhance the immune response against tumours and can be used in combination with other treatments for improved effectiveness.
What are the different types of cancer vaccines
Cell-based vaccines
Created using patient’s
own cancer cellsActivated cancer cells
delivered back to
patient with other
proteins (e.g. IL-2)
leading to immune
activation
Vector-based vaccines
Engineered virus or
other vector is used to
introduce carry specific
proteins to recognize
cancer cells and fight
against
Antigen vaccines
Proteins or peptides
that are antigenic are
produced using
recombinant strategies,
purified and
administered
What are the cons of cancer vaccines
(majority are similar to Mabs)
Cancer vaccines are targeted; mutations in cancer cells would reduce the efficiency (e.g. due to loss of target antigens on cancer cell surface)
Cancer antigens may not be same in different patients and hence cancer vaccines are not effective in all cases
Manufacturing challenges, expensive
What are the pros of cancer vaccines
Cancer vaccines stimulate the immune system to specifically target and attack cancer cells, potentially leading to long-lasting immunity.
They can also be used as an adjunct to other therapies, improving overall treatment outcomes.
What are plasma cells and its purpose
Differentiated from B-cells/B-lymphocytes made in the bone marrow
Secrete antibodies into the blood or other body fluids
What is multiple myeloma
A cancer of plasma cells within their late stages of differentiation
Characterised by accumulation of plasma cells in the bone marrow
The myeloma cell is a post-geminal centre plasma cell that has undergone Ig class switching and somatic hypermutation
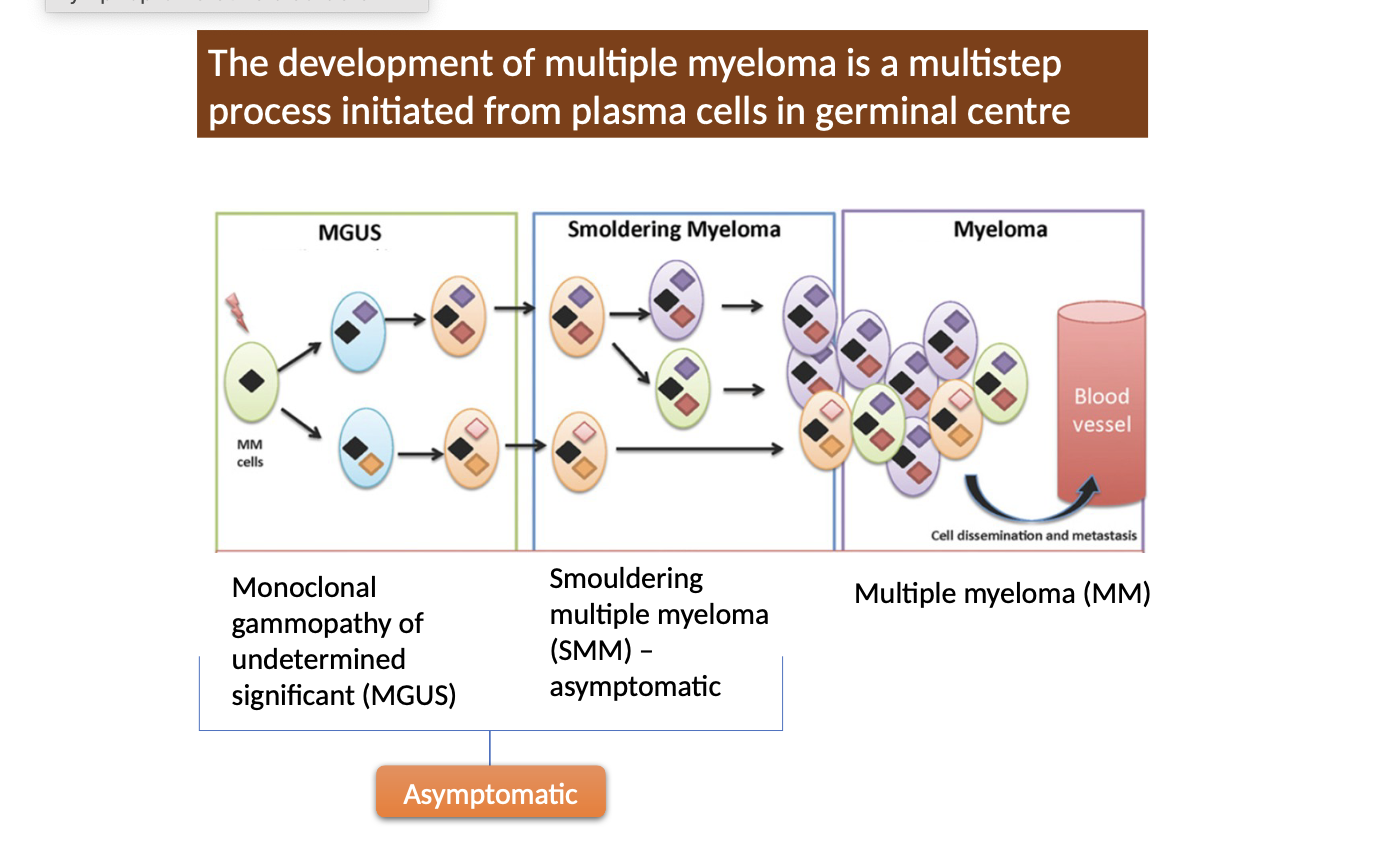
What are the different development stages of myeloma
Multiple myeloma develops through stages with increasing severity and clinical manifestations
monoclonal gammopathy of undetermined significance (MGUS)
smouldering multiple myeloma,
symptomatic multiple myeloma
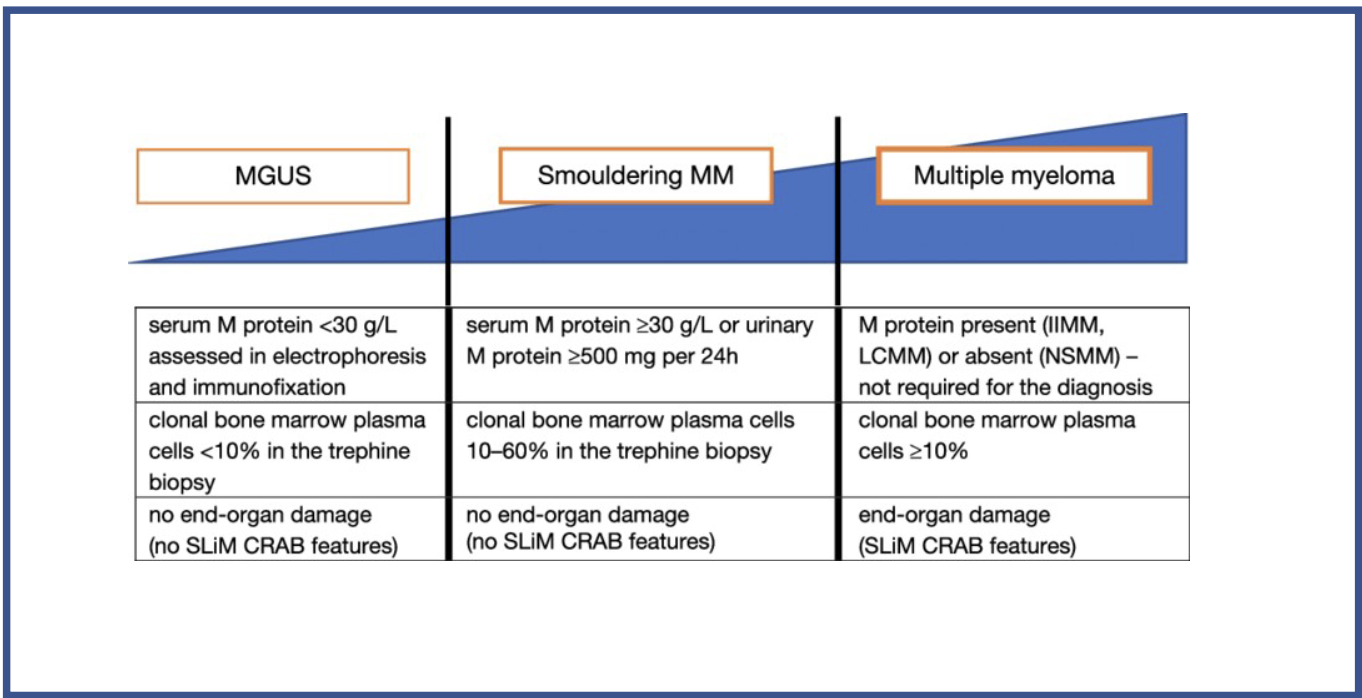
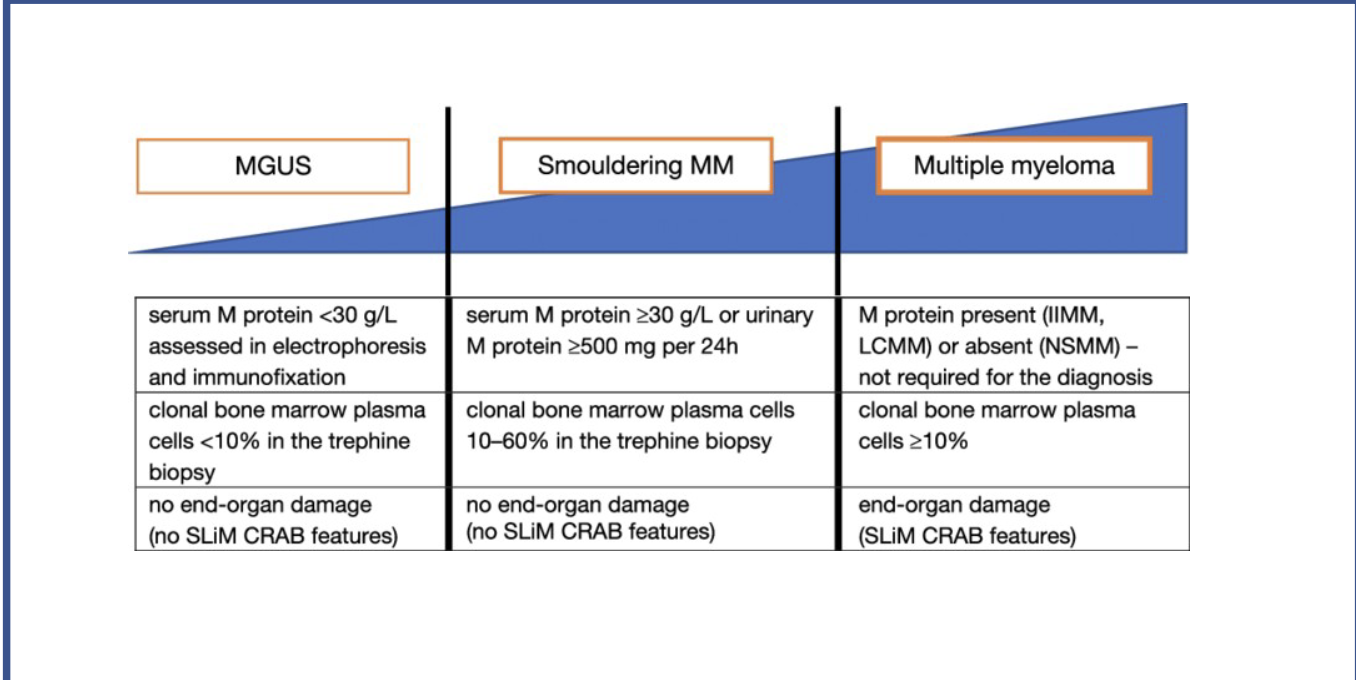
What test results are needed for each stage
MAGUS:
Serum M protein <30g/L
Clinical bone marrow plasma cells <10% in trephine biopsy
No end-organ damage (no slim C.R.A.B features)
Smouldering MM:
Serum M protein >30g/L, or urinary M protein>500mg in 24hrs
Clinical bone marrow plasma cells 10%-60% in trephine biopsy
No end-organ damage (no slim C.R.A.B features)
MM:
Serum M protein present
clonal bone marrow plasma cells >10%
evidence of end-organ damage (presence of slim C.R.A.B features).
What are the two groups of myeloma relating to genetics abnormalities
Non-hyperdiploid group
A translocation involving IGH locus on chromosome 14
Recurrent translocation partners – 4, 6, 11,16 or 20
Hyperdiploid group
Trisomies of chromosome 3, 5, 7, 9, 11, 15, 19 and 21
What does the genetic abnormalities do
Genetic abnormalities alter the expression of adhesion molecules
Myeloma cells adhere to bone marrow stromal cells --> induces cytokines and growth factors release [Survival, Migration, and drug resistance]
Myeloma cells adhere to extracellular matrix protein --> inhibits apoptosis
IL-3
What are the clinical manifestations of myeloma C.R.A.B
HyperCalcaemia
Renal impairment
Anaemia
Bone disease
How does hypercalcaemia link to the pathogenesis MM
Myeloma cells release substances like RANKL (Receptor Activator of Nuclear Factor κB Ligand), which activates osteoclasts.
Increased in Osteoclast activity
Release of calcium in to the blood
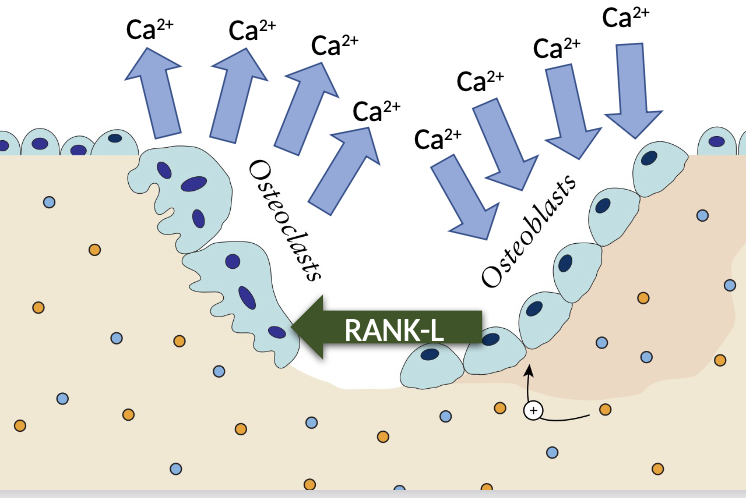
How does renal impairment link to the pathogenesis MM
Hypercalcaemia leads to calcium depositing in the kidney
Light chains and monoclonal antibodies causing renal failure electrolyte imbalance
Decrease in the production of erythropoietin (EPO)
How does anemia link to the pathogenesis MM and its symptoms
Present in >60% of patience
Shift in the production of cells from myeloid to lymphoid to make more plasma cells
In MM, cancerous plasma cells multiply and take up space in the bone marrow, where normal blood cells (including red blood cells) are made.
MM often causes renal impairment due to light chain deposition.
Damaged kidneys make less erythropoietin (EPO), a hormone needed to make red blood cells.
Less EPO = less red blood cell production = anaemia.
Symptoms include:
Shortness of breath
Fatigue
Pale appearance
Light-headedness
How does bone disease link to the pathogenesis MM and its symptoms
Seen in more than 50% of cases
Occurs due to over production of osteoclast activity
Bone lesions commonly found in ribs and spine, causing back pain
Leads to fractures and pain
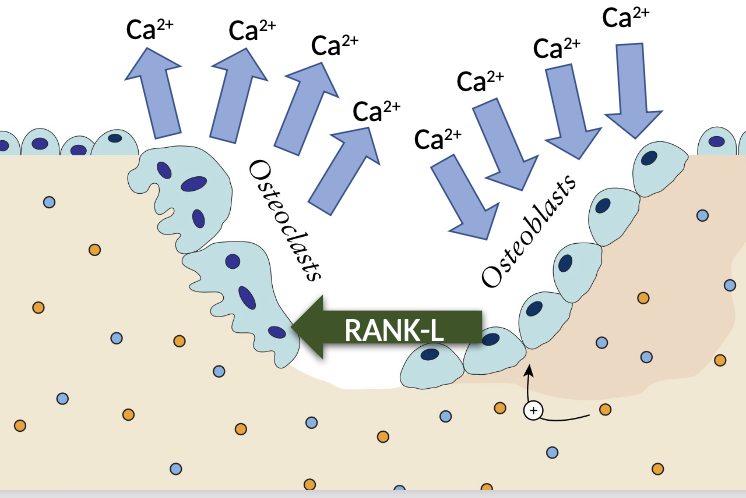
What are osteoblasts and osteoclasts
Osteoblasts are cells responsible for bone formation
Osteoclasts are cells that break down bone tissue.
They play crucial roles in bone remodelling and maintaining bone health.
What can hypercalcemia lead to
Hypercalcemia can lead to a number of health conditions, including:
Kidney stones: Calcium crystals can form in the kidneys and eventually combine into stones.
Kidney failure: Over time, hypercalcemia can damage the kidneys, limiting their ability to filter blood and remove excess fluid.
Osteoporosis: Increased calcium in the bones leads to fragile bones and can lead to a loss of height and curving of the spine.
Nervous system conditions: Severe hypercalcemia can lead to confusion, dementia, and coma.
Hypertension: This can be a complication of hypercalcemia.
How does bone disease pathogenesis link to myeloma (ExtraReading)
How do we diagnose for myeloma the 4 types
Bone marrow biopsy or aspiration
Blood testing
Urine testing
Bone testing
X-ray - lytic lesions, thinning of bone, negative in 25%, the gold standard
MRI - Used when X-rays are negative, reveals the presence and distribution of MM
CT - Useful for small areas of evaluation
PET/CT - Sensitive, whole body, disease monitoring
myeloma includes several methods: a bone marrow biopsy to assess abnormal plasma cells, X-rays to identify lytic bone lesions, MRI when X-rays are inconclusive, and various blood and urine tests for monoclonal proteins and kidney function.
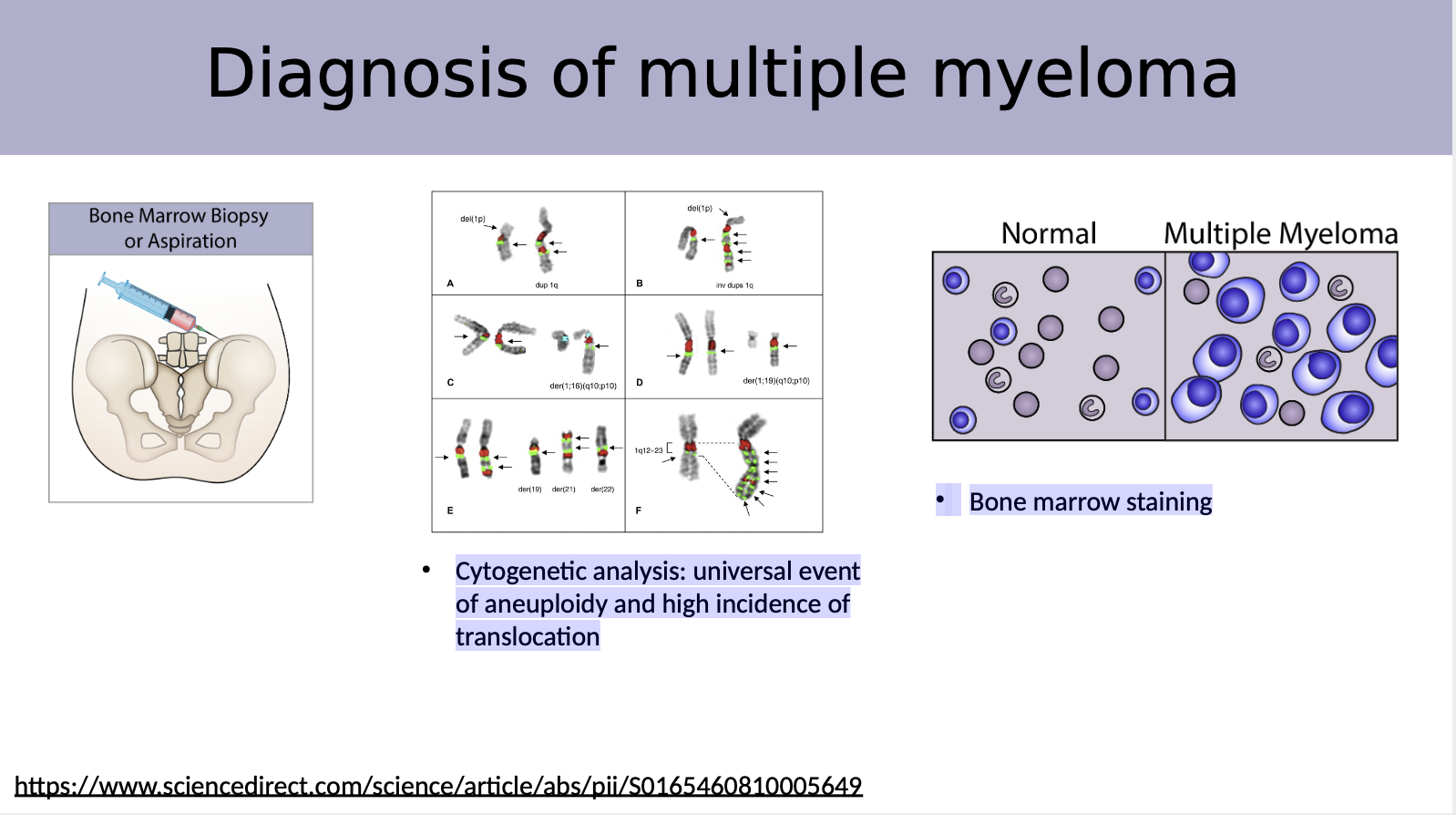
Explain why bone marrow biopsy is a diagnosis method
Cytogenetic analysis: universal event of aneuploidy and high incidence of translocation
Bone marrow staining helps identify abnormal cells and assess the bone marrow's overall health.
Explain why urine testing is a diagnosis method
Presence of a paraprotein via immunoglobulin electrophoresis
• IgG account for 60% of cases
• IgA account for 20% of cases
Elevated serum Ig-free light chains (FLC)
• FLC are K and λ - unpaired and synthesised by plasma cells
• Increased K or λ value
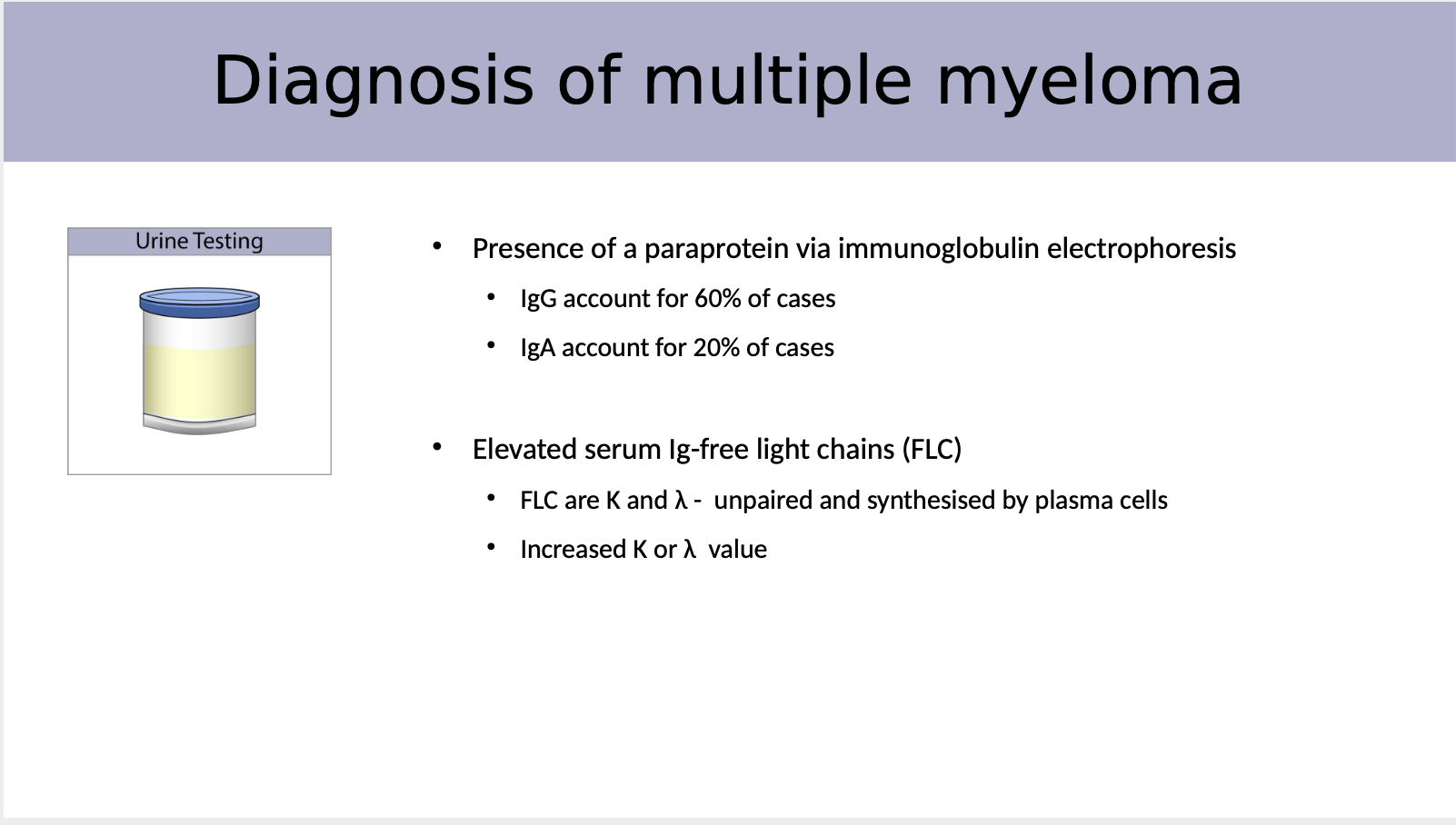
The amount of what can differ in the location of the body
Explain why blood testing is a diagnosis method and what we can diagnose
Presence of a paraprotein via immunoglobulin electrophoresis
IgG account for 60% of cases
IgA account for 20% of cases
Elevated serum Ig-free light chains (FLC)
FLC are K and λ < unpaired and synthesised by plasma cells
Increased K or λ value
Decreased level of normal IgG, IgA and IgM: Immunoparesis
[suppression of polyclonal immunoglobulins]
Elevated calcium level in serum
Graph:
Changes in serum protein electrophoresis
Elevated serum β2-microglobulin
A large spike in gamma region
![<ol><li><p><strong>Presence of a paraprotein via immunoglobulin electrophoresis</strong></p><ul><li><p>IgG account for 60% of cases</p></li><li><p>IgA account for 20% of cases</p></li></ul></li><li><p><strong>Elevated serum Ig-free light chains (FLC)</strong></p><ul><li><p>FLC are K and λ < unpaired and synthesised by plasma cells</p></li><li><p>Increased K or λ value<br></p></li></ul></li><li><p><strong>Decreased level of normal IgG, IgA and IgM: Immunoparesis</strong></p><ul><li><p>[suppression of polyclonal immunoglobulins]</p><p></p></li></ul></li><li><p><strong>Elevated calcium level in serum</strong><br></p></li><li><p><strong>Graph: </strong></p><ul><li><p>Changes in serum protein electrophoresis</p></li><li><p>Elevated serum β2-microglobulin</p></li><li><p>A large spike in gamma region</p></li></ul></li></ol><p><br></p>](https://knowt-user-attachments.s3.amazonaws.com/b5000a41-143f-4815-bbbb-505dd299f733.png)
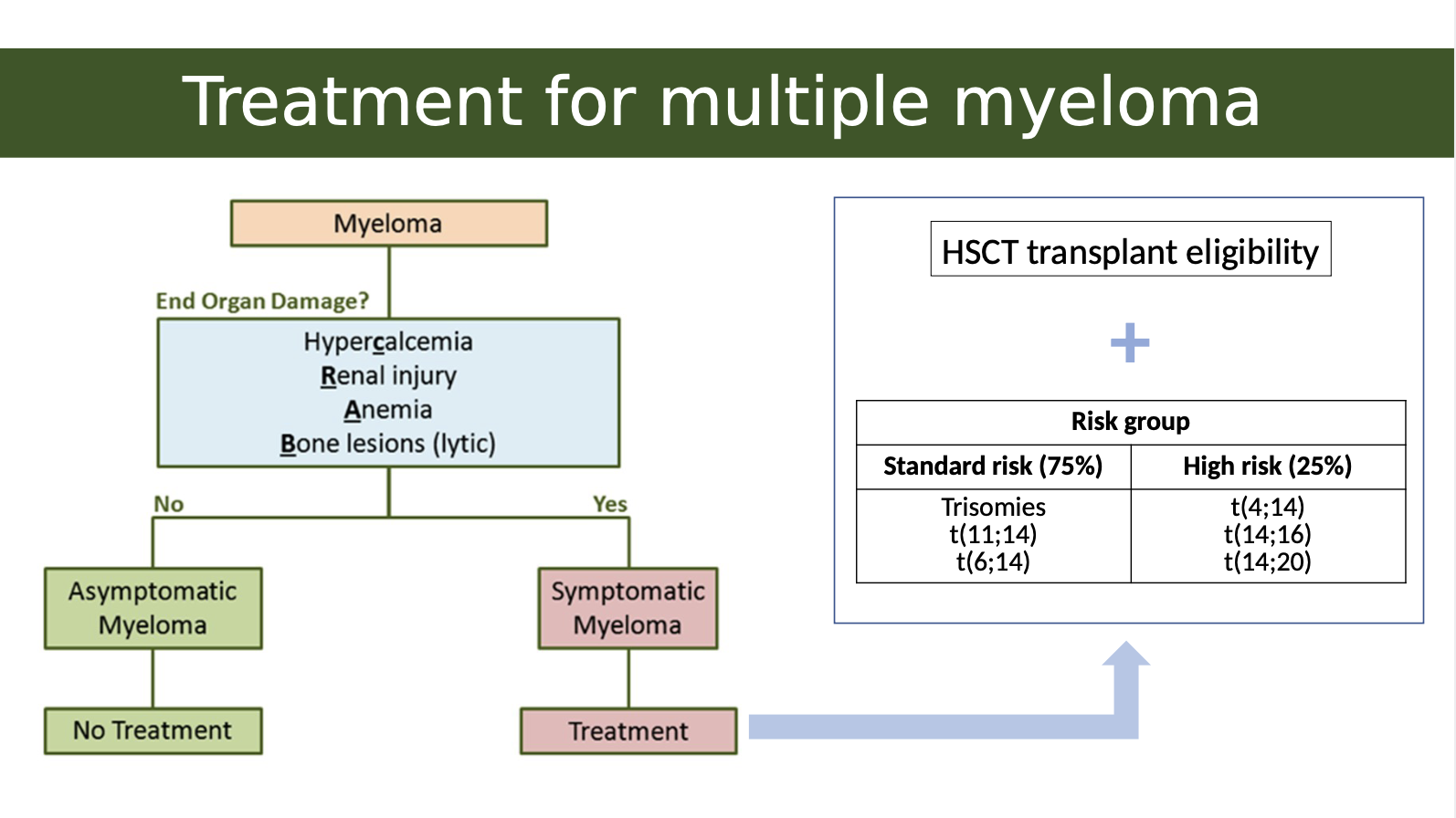
What is the treatment for multiple myeloma
The treatment for multiple myeloma typically involves high-dose chemotherapy followed by hematopoietic stem cell transplantation (HSCT), which can significantly improve patient outcomes.
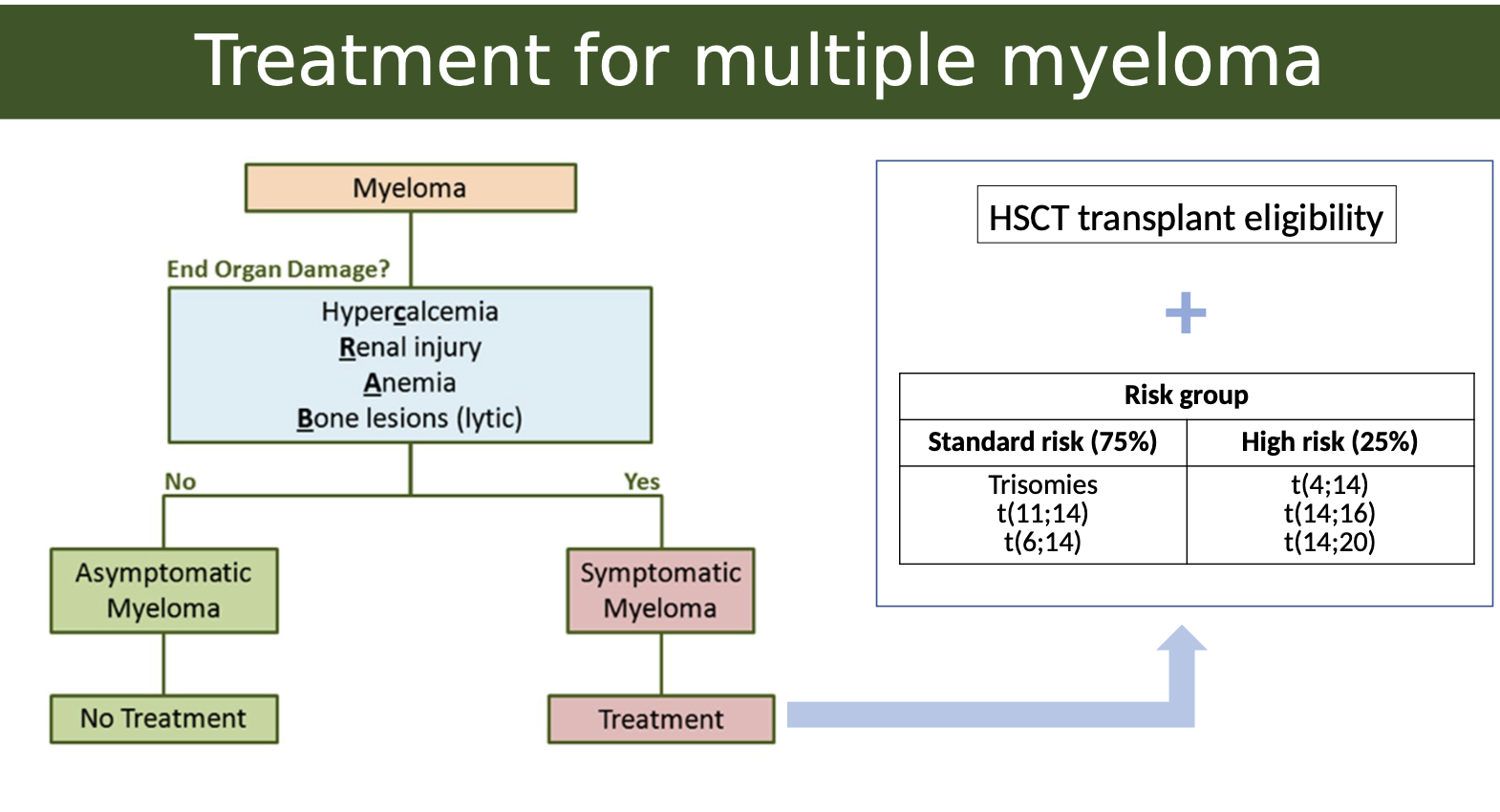
What is the link between genetics and multiple myeloma treatment
In Multiple Myeloma, genetic mutations or translocations help determine how aggressive the disease is. These are grouped into:
Standard Risk (~75%)– Better prognosis
These patients tend to respond well to treatment.
Common cytogenetic features:
Trisomies: Extra copies of certain chromosomes (usually odd-numbered like 3, 5, 7, 9, 11, 15, 17, 19, 21).
t(11;14) and t(6;14): Translocations involving chromosome 14 (immunoglobulin heavy chain gene) and chromosomes 11 or 6 — associated with less aggressive disease.
High Risk (~25%)– Worse prognosis
These patients may be more resistant to treatment or relapse earlier.
Key high-risk translocations:
t(4;14)
t(14;16)
t(14;20)
These all involve IgH gene (on chromosome 14) fused with oncogenes, leading to more aggressive cancer behaviour.
What is lymphoma
Lymphoma is a cancer of the lymphatic system, caused by abnormal growth of lymphocytes.
It affects your body’s ability to fight infections and can spread through the lymphatic system.
Malignancy of lymphocytes
Accumulates in lymph nodes
Other lymphoid organs/tissue
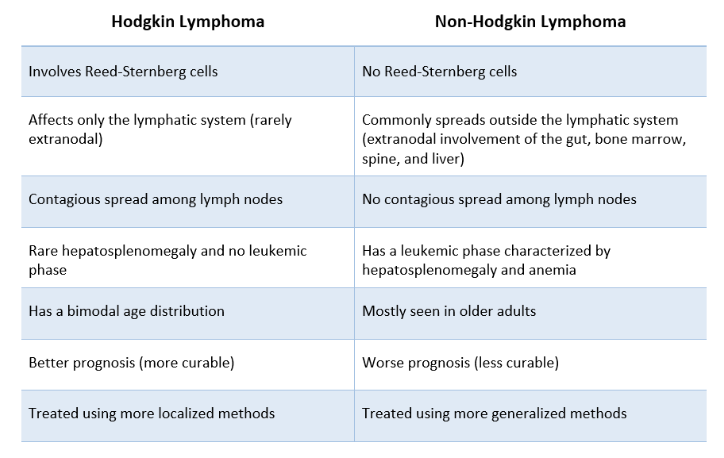
What is the difference between Hodgkins and non-Hodgkins lymphoma
Hodgkin's lymphoma is characterized by the presence of Reed-Sternberg cells and typically presents with a predictable pattern of spread,
Non-Hodgkin's lymphoma includes a diverse group of malignancies that do not have Reed-Sternberg cells and may spread unpredictably.
What are the clinical manifestations of lymphoma
A painless lymph node mass
Constitutional symptoms:
Recurrent fevers
Night sweat
Significant weight loss
Moderate splenomegaly
How is lymphoma diagnosed
Blood test:
Complete blood count (CBC)
Increased erythrocyte sedimentation rate (ESR)
Increased C-reactive protein (CRP) level
Increased Lactate dehydrogenase (LDH) level
Excisional biopsy
The initial diagnosis of NHL is carried out through the excisional biopsy of a suspicious lymph node or tumour in another organ
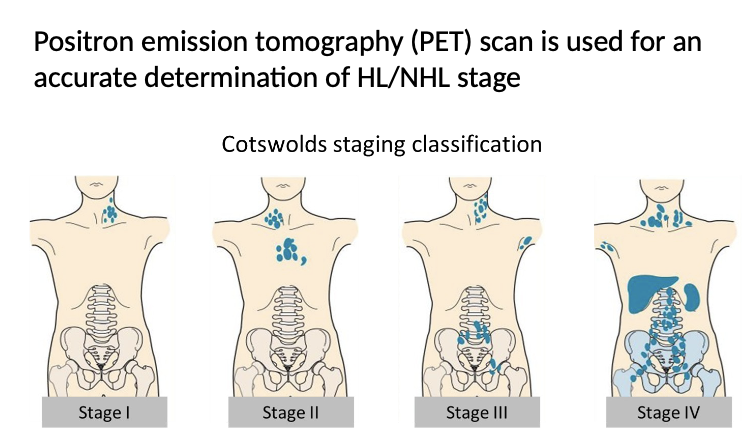
PET Scan
Positron emission tomography (PET) scan is used for an accurate determination of HL/NHL stage
The stage of disease always followed by A (absent) or B (present) for constitutional symptoms:
Fever with the body temperature above 38 °C
Night sweat
Loss of more than 10 % of the body weight in the past 6 months