Unit 1 Functional Groups
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Three main types of functional groups:
Hydrocarbons (molecules containing only C and H)
Compounds containing a C-Z σ bond where Z = an electronegative atom
Compounds containing a C=O group
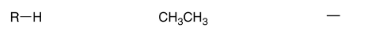
Alkane
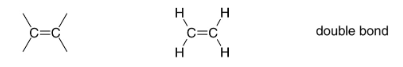
Alkene
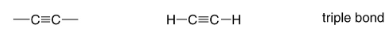
Alkyne
System of pi bonds
Aromatic compound (Arene is exclusively hydrocarbons)
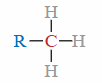
Primary (1°) Carbon
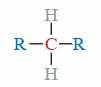
Secondary (2°) Carbon
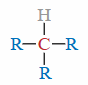
Tertiary (3°) Carbon
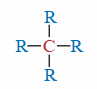
Quaternary (4°) Carbon

SP3 Carbon
Alkyl halide
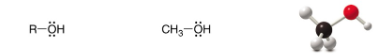
SP3 Carbon
Alcohol
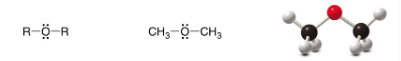
SP3 Carbon
Ether
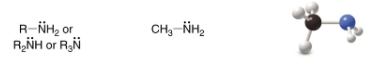
SP3 Carbon
Amine
SP3 Carbon
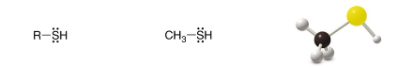
Thiol
SP3 Carbon
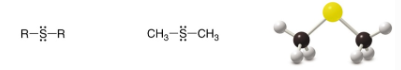
Sulfide
SP2 Carbon
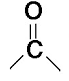
Carbonyl
SP2 Carbon
(Ketone at the end of a molecule)
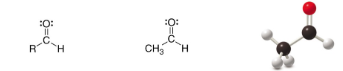
Aldehyde
SP2 Carbon
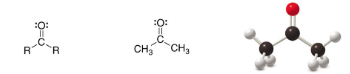
Ketone
SP2 Carbon
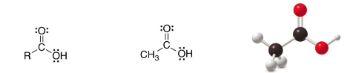
Carboxylic acid
SP2 Carbon
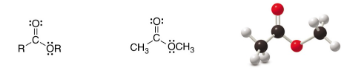
Ester
SP2 Carbon
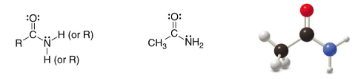
Amide
SP2 Carbon
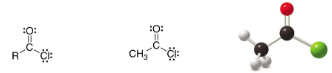
Acid chloride
meth (methane) number of carbons
1 Carbon
eth (ethane) number of carbons
2 Carbons
prop (propane) number of carbons
3 Carbons
but (butane) number of carbons
4 Carbons
pent (pentane) number of carbons
5 Carbons
hex (hexane) number of carbons
6 Carbons
hept (heptane) number of carbons
7 Carbons
oct (octane) number of carbons
8 Carbons
non (nonane) number of carbons
9 Carbons
dec (decane) number of carbons
10 Carbons
undec (undecane) number of carbons
11 Carbons
dodec (dodecane) number of carbons
12 Carbons
tridec (tridecane) number of carbons
13 Carbons
tetradec (tetradecane) number of carbons
14 Carbons